Infection prevention in Endoscopy is gaining more and more attention. Every year tens of millions of patients benefit from endoscopic procedures. Improper cleaning of endoscopes may lead to failure of high-level disinfection and sterilization procedures and may increase the potential risk for infection transmission to patients1. Boston Scientific is committed to the entire continuum of patient care including products and training designed to help our customers meet or exceed industry guidelines for infection prevention within the GI space.
Scope of the Problem
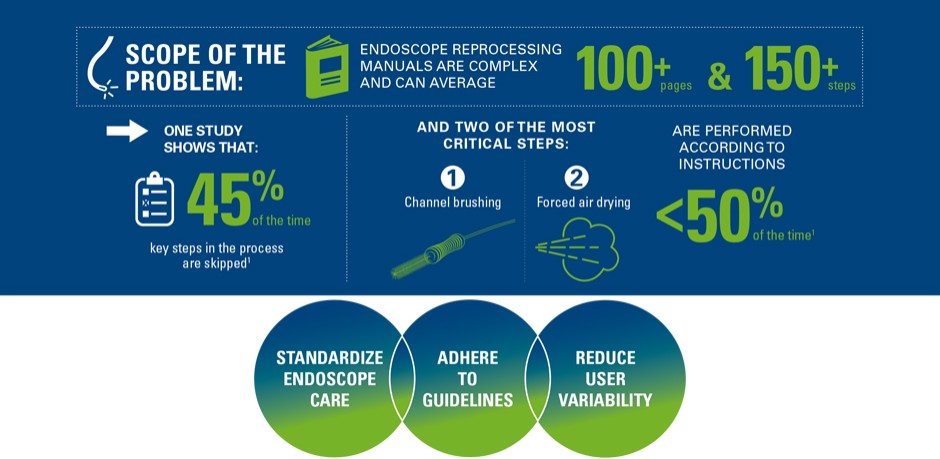
Endoscope reprocessing manuals are complex and can be in average more than 100 pages and more than 150 steps. Consequently, studies show that key steps are skipped in the cleaning process 45% of time and that two of the most critical steps, manual cleaning and drying are performed according to instructions less than 50% of time.1
How much do you really know about Infection Prevention issues? Test your knowledge.
While 99%+ of these cases were not found in peer-reviewed medical journals2, more and more clinical evidence is available about infection issues. How well do you know studies around infection prevention?
Enhance your knowledge on the most current information on infection risks associated with endoscopy and actions you can put in place to minimize them. Watch on demand recordings of Boston Scientific’s educational webcast series at you leisure.

The Boston Scientific Infection Prevention portfolio of products is designed to provide the components needed to comply with industry guidelines, mitigate cross-contamination risk, and reduce variablility during endoscope reprocessing.

















