Reprocessing is complex, time-sensitive, and resource-intensive
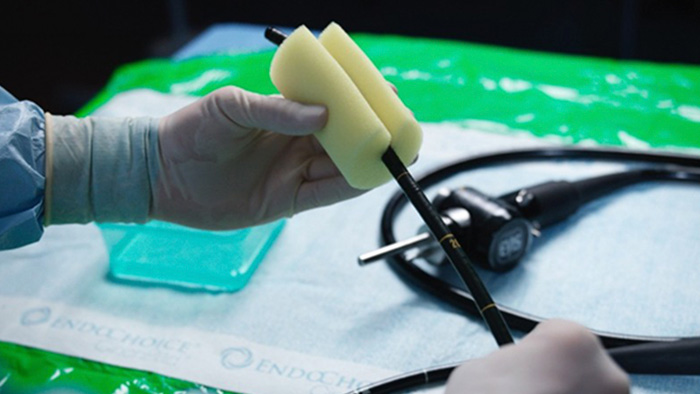
Manual cleaning of the endoscope must start immediately after it has been withdrawn from the patient
The system must then be transported to the reprocessing area, where manual cleaning should start within ~30 minutes.¹
Duodenoscope reprocessing manuals can average >100 pages and >150 steps.²
Preparing the scope for re-use is demanding, requiring the time and dedication of trained staff and thereby taking them away from more direct forms of patient care.
It is also ineffective
In a study, only 1.4% of trained staff were found to perform the reprocessing steps correctly.³
15% of patient-ready scopes were found to be contaminated in a nationwide study in the Netherlands that tested >150 duodenoscopes at >70 centers.⁴
In a 2015 outbreak of drug resistant KI. pneumoniae (MRKP) in a Netherlands medical center, two duodenoscopes were responsible for attack rates* of 35% and 29% respectively.⁵
*Attack rate: Number of infected or colonized cases/number of exposed persons.
Postprocedural control can be as simple as disposing of a single-use scope

The Boston Scientific single-use portfolio eliminates duodenoscope reprocessing servicing and repairs.
It also minimizes the risk of infection and its associated costs.
All your department will need is a biohazard waste disposal protocol.
Facilitating infection prevention in the HPB (hepato-pancreato-biliary) pathway
At Boston Scientific, we understand the array of obstacles you face in ensuring that your patients undergoing ERCP avoid infection. That is why we have developed solutions for every step in the process.


















