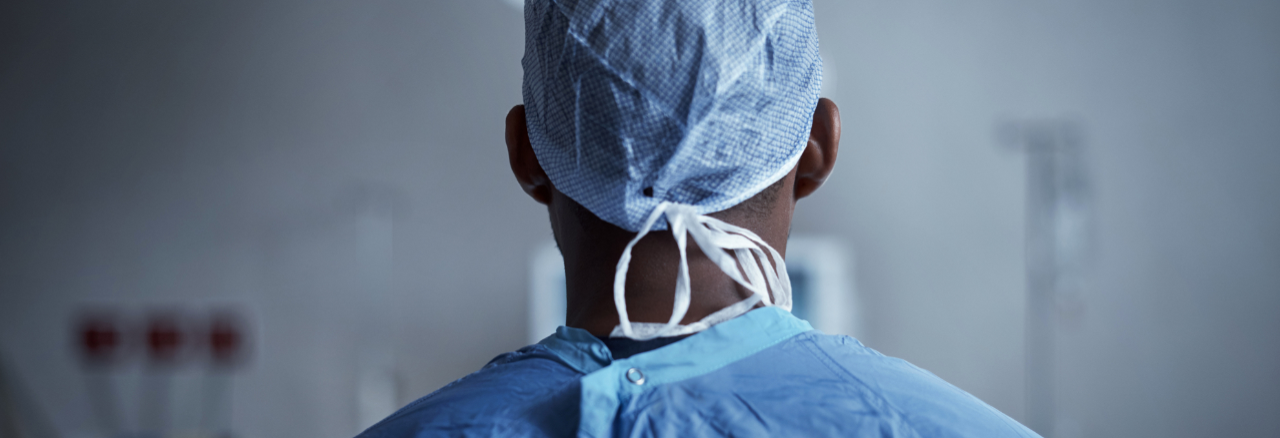
Time Course of HF Decompensation¹
Emulating Clinical Assessment via Physiologic Sensor Monitoring
Electrophysiology Insights
HeartLogic uses automated intelligence to streamline your ability to see each patient’s physiologic response to arrhythmic and pacing changes.
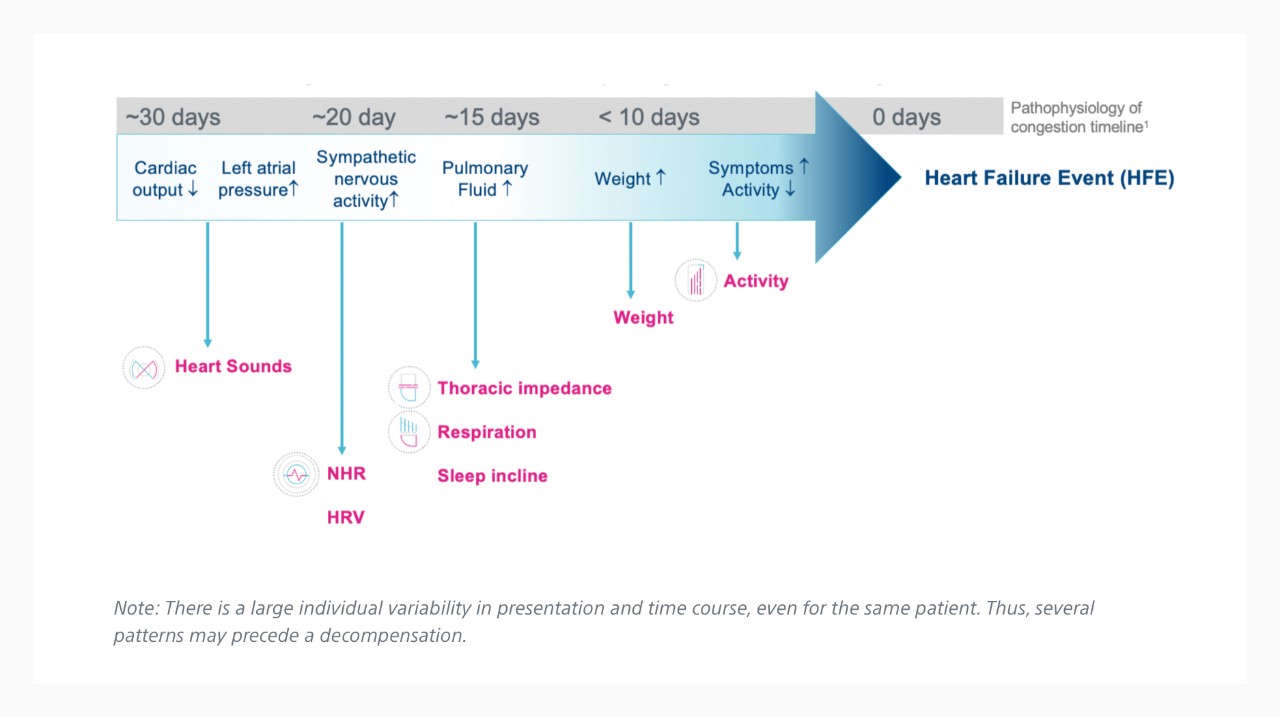
Relationship Between Atrial Fibrillation and HeartLogic Index²
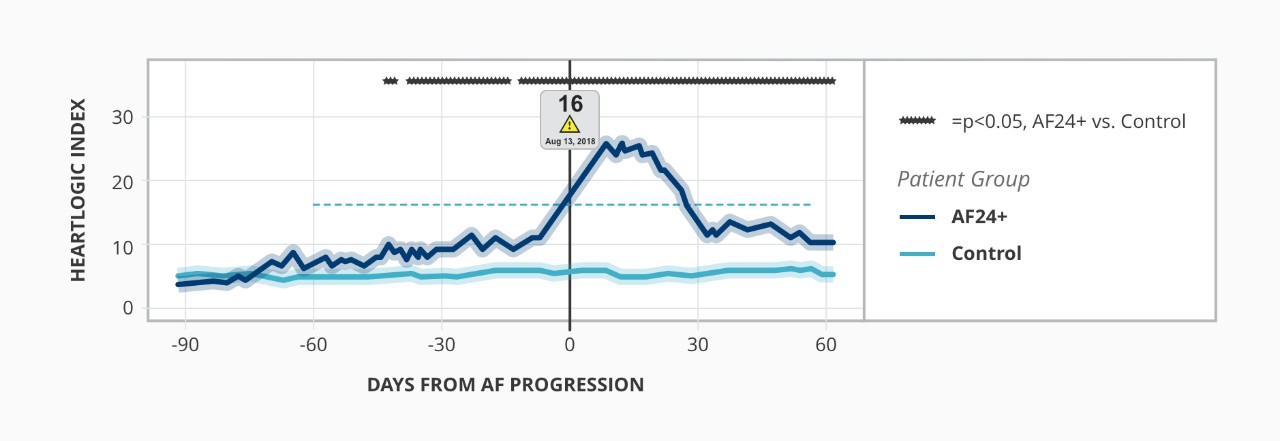
HeartLogic May Detect Sub-Optimal BiV Pacing and Poor Physiologic Response³
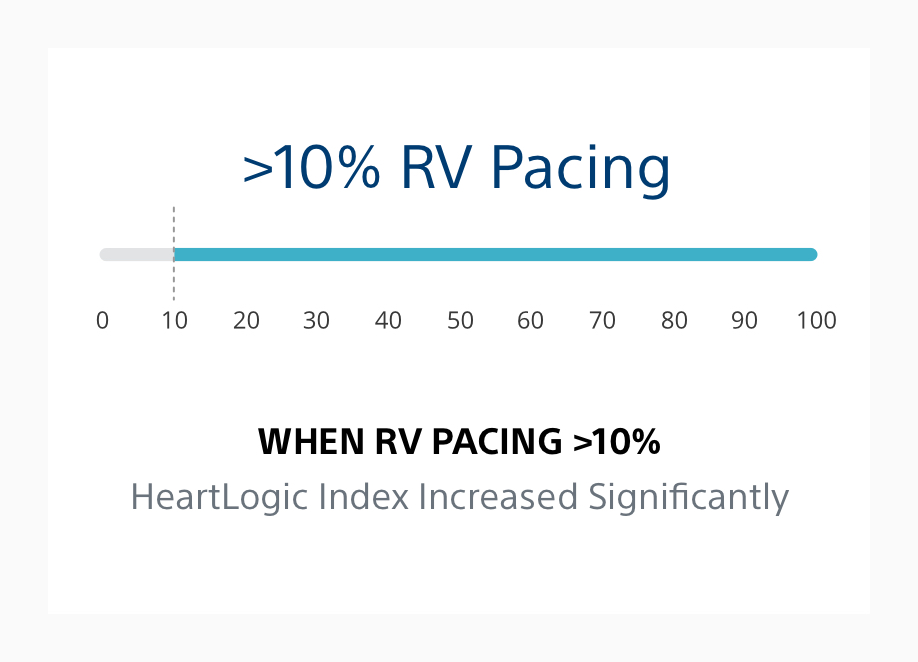
HeartLogic May Identify Patients Vulnerable to RV Pacing⁴
MANAGE-HF Study⁵
Phase I of the MANAGE-HF study enrolled 200 patients implanted with a CRT-D or ICD enabled with HeartLogic. The study found that HeartLogic was safely integrated into clinical practice and associated with lower natriuretic peptide levels and hospitalization rates.⁶
MORE RAPID RECOVERY
Of HeartLogic Index
LOWER
NTproBNP Levels
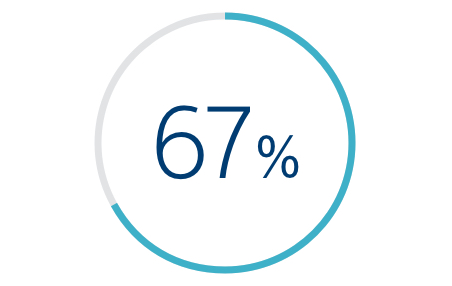
67% REDUCTION
In HF Hospitalizations Associated with HeartLogic*
*Compared with pre-study hospitalization rate (12 months).
MultiSENSE Study Results⁷
The MultiSENSE study assessed more than 900 patients and validated that the HeartLogic algorithm provides a sensitive and timely predictor of impending heart failure decompensation.

70% SENSITIVITY
in Detecting Heart Failure Events
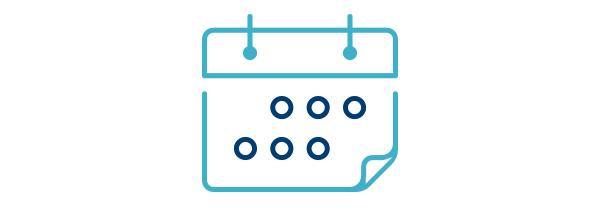
A MEDIAN OF 34 DAYS
Advance Notice of Worsening Heart Failure

<2 TOTAL ALERTS
Per Patient Per Year
Real-World Evidence
In real-world analyses of nearly 500 patients across four studies, HeartLogic was shown to provide consistent heart failure detection performance with low unexplained alert rates.
Real-World Results Compared to Validation Data Set
| SENSITIVITY | UNEXPLAINED ALERT RATE | |
| MultiSENSE⁷ (Validation Data Set) | 70% | 1.47 |
| Capucci et al.² (ESC HF 2019) | 100%* | 0.41 |
| Santini et al.⁸ (Clin Card 2020) | 69%* | 0.37 |
| RE-HEART Phase I⁹ (ESC HF 2020) | N/A | 0.25 |
| RE-HEART Phase II¹⁰ (ESC HF 2020) | N/A | 0.13 |
Highlights from Real-World Studies
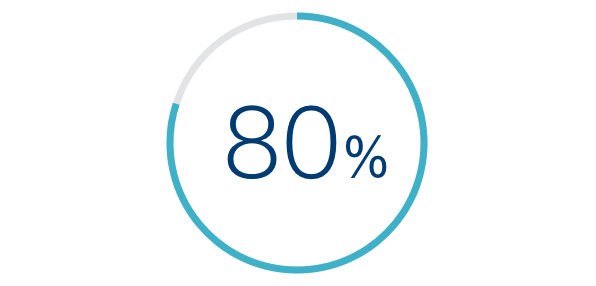
80% OF ALERTS
Provided New Information to Clinicians⁸

ALERTS PRECEDED HF SYMPTOMS
by a Median of 12 Days²

ALERTS PRECEDED HF EVENTS
by a Median of 38 Days²
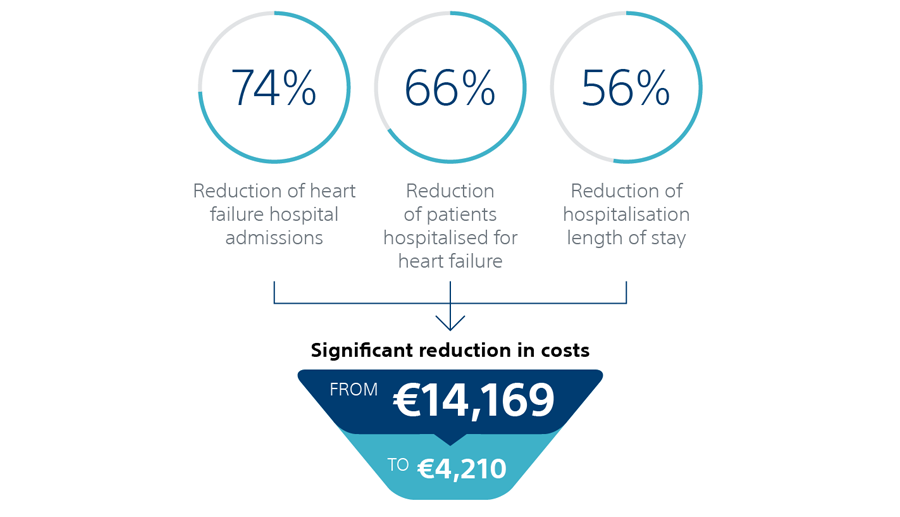
HeartLogic reduces hospitalisations for decompensated heart failure and significantly reduces the costs per patient for the healthcare system, as described in the Heggermont et al publication.¹¹
Results from APAF-Mortality Clinical Trial CRT Ablate and Pace – Proven Best Option
Professor Michele Brignole and Isabelle van Gelder reviewed clinical data coming from the APAF-CRT Mortality trial which has made very clear that Ablation + CRT was superior to pharmacological therapy in reducing mortality in patients with permanent AF.
These new and powerful results strengthens and supports the CRT therapy also for these indicated patients.