Clinical Evidence
Numerous studies have demonstrated the safety and efficacy of the POLARx™ Cryoablation System in the treatment of paroxysmal atrial fibrillation. It has been studied and is in use in multiple countries across the globe.

For health care professionals in EUROPE except those practicing in France as the following pages are intended to all International health care professionals and are not in compliance with the French Advertising law N°2011-2012 dated 29th December 2011 article 34. Other health care professionals should select their country in the top right corner of the website.
Please note that the following pages are exclusively reserved for health care professionals in countries with applicable health authority product registrations. To the extent this site contains information, reference guides and databases intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions.
This Website is protected by the laws on copyright and by the relevant international conventions. It is strictly forbidden to make copies, whether partial or total and on whichever media without prior approval.
Numerous studies have demonstrated the safety and efficacy of the POLARx™ Cryoablation System in the treatment of paroxysmal atrial fibrillation. It has been studied and is in use in multiple countries across the globe.
***Updated analysis with corrected data
| Procedural Characteristic | FROzEN-AF (28 mm balloon) | POLARx FIT Extension Arm (28 mm / 31 mm balloon) |
| General Anesthesia (%) | 78.5% | 100% |
| Conscious Sedation/MAC (%) | 21.5% | - |
| Procedure Time (min) | 91 ± 41 min | 101 ± 59 min |
| LA Dwell Time (min) | 59 ± 33 min | 51 ± 22 min |
| Fluoroscopy Time (min:sec) | 12:52 ± 11:12 min | 7:10 ± 11:18 min |
| Grade 3-4 Occlusion* (%) | 95.9% (69.9% - Grade 4) | 97.7% (66.4% / 77.6% - Grade 4) |
| Single Shot Success* (%) | 55.9% | 35.3% / 62.1% |
Mean ± SD
*Only ablations with duration >60 sec included in ablation counts
FROZEN AF (POLARx)¹
(n=325)
12-MONTH EFFICACY
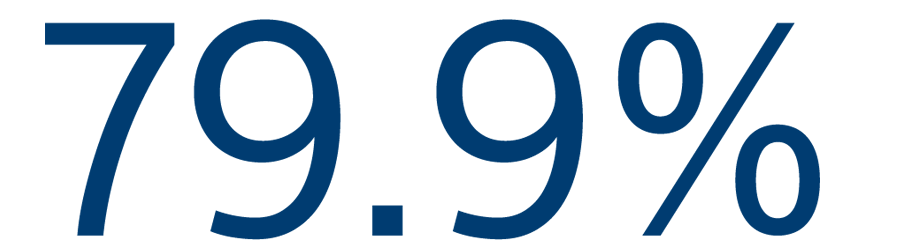
Freedom from atrial arrhythmias
12-MONTH SAFETY
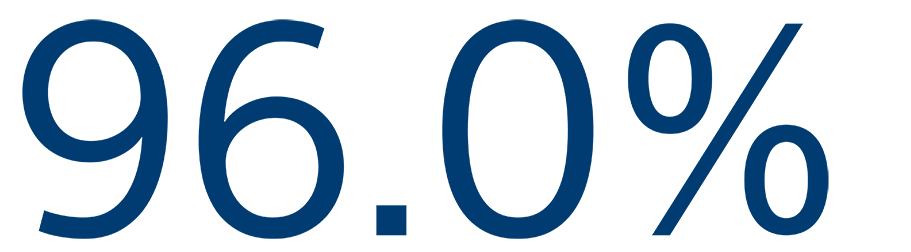
Freedom from primary event
Extended POLARx FIT arm1
(n=50)
12-MONTH EFFICACY

Freedom from atrial arrhythmias
12-MONTH SAFETY
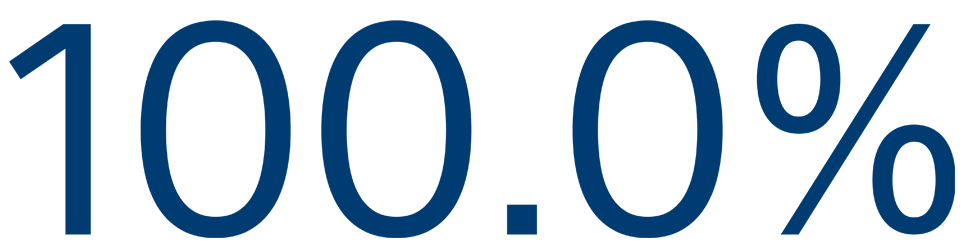
Freedom from primary event**
Procedural Characteristics | Overall (n=24) |
Overall procedural time (min), median (IQR) | 90 (60–110) |
Overall fluoroscopy time (min), median (IQR) | 15.5 (12–22.3) |
Anatomical variants Right common pulmonary trunks, n (%) Left common pulmonary trunks, n (%) Additional PV branches, n (%) |
2 (8.3) 1 (4.2) 3 (12.5) |
Number of applications needing the 31 mm size, n (%) | 64 (51.6) |
Minimal reached temperatures LSPV (°C), median (IQR) LIPV (°C), median (IQR) RSPV (°C), median (IQR) RIPV (°C), median (IQR) |
-52.0 (−54.5–−50.5) -49.0 (−52.0–−48.0) -50.0 (−54.0–−45.5) -50.0 (−57.0–−48.0) |
In-hospital AF recurrences, n (%) | 2 (8.3) |
Periprocedural complications Groin hematoma, n (%) Pericardial effusion, n (%) Cardiac tamponade, n (%) Phrenic nerve palsy, n (%) Thromboembolic complications, n (%) |
1 (4.2) 0 (0) 0 (0) 0 (0) 0 (0) |
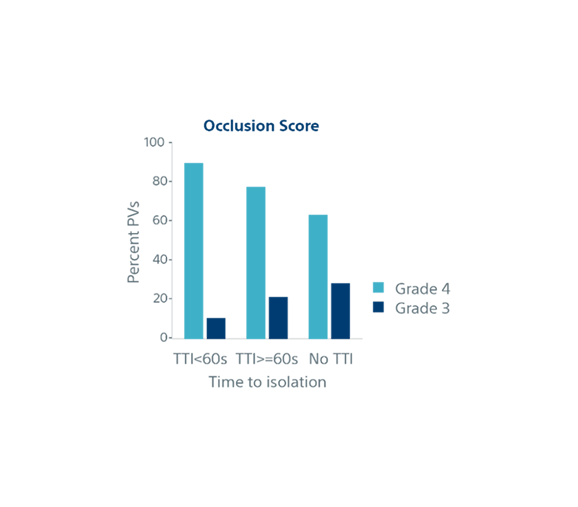
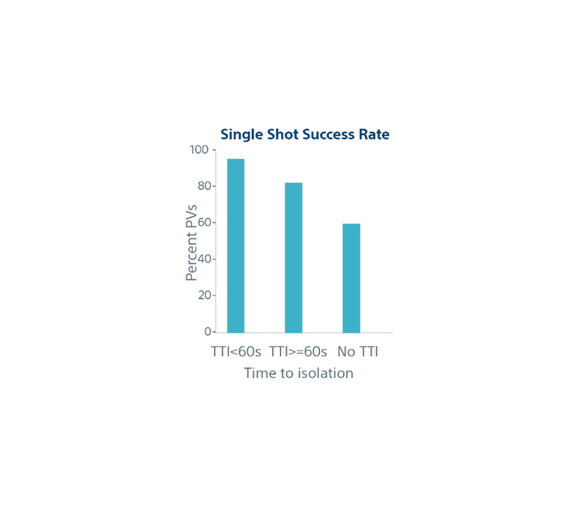
| Biophysical parameters and single shot success | TTI <60s (n=852) | TTI ≥60s (n=282) | No TTI (n=776) | p-value |
| Time to -40°C (s) | 30.6 ± 7.4* | 34.2 ± 10.7 | 35.0 ± 15.3 | p<0.001 |
| Nadir Temperature (°C) | -58.3 ± 5.8 | -56.6 ± 5.9 | -54.1 ± 6.8 | p<0.001 |
| Thaw to °C (s) | 21.1 ± 6.7 | 19.3 ± 5.5 | 17.6 ± 6.7 | p<0.001 |
| Occlusion Grade (4) | 89% | 77% | 63% | p<0.001 |
| Occlusion Grade (3) | 11% | 22% | 29% | |
| Single Shot Rate | 95% | 82% | 60% | p<0.001 |
Data reported when available. Not all ablations reported full data, results are mean + SD or percentage.
*Significantly less than the other 2 groups.
Procedural characteristics⁴
| Procedural characteristics⁴ | |
| Mean procedure time | 68.2 min |
| Mean left atrial dwell time | 46.6 min |
| Mean fluoroscopy time | 15.6 min |
Performance and biophyscial characteristics⁴
| Performance and biophysical characteristics⁴ | |
| Acute pulmonary vein isolation | 96.80% |
| Grade 3-4 occlusion | 98.20% |
| Single-shot success | 71.20% |
| Mean cryoablation per PV | 1.5 |
| Mean nadir temp | -56.3°C |
| Mean time to isolation | 50 sec |
Safety⁵

Primary safety event rate

Major advers events No patient suffered from atrial esophageal fistula or pulmonary vein stenosis
12-month efficacy⁵
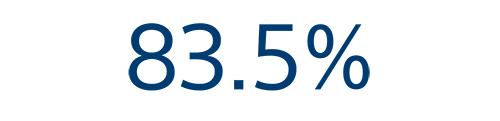
Arrhythmia recurrence free rate
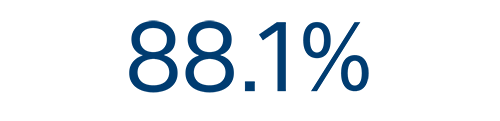
AF
recurrence free rate
| Performance and biophysical characteristics⁶ | |
| Acute pulmonary vein isolation | 99.70% |
| Real-time PVI visualization | 71.90% |
| Minimum CB temperature | −57.9±7°C |
| Precedure time | 92±41 min |
| Fluoro time | 15±10 min |

Freedom from
recurrence
at 226 ± 115 days
| Multi-center | Single-center |
| Spera, et al, 2022⁷ Ultra-high-resolution assessment of lesion extension after cryoballoon ablation for pulmonary vein isolation | Anic, et al 2021¹¹ Acute safety, efficacy, and advantages of a novel cryoballoon ablation system for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: initial clinical experience |
| Fassini, et al., 2022⁸ Novel cryoballoon technology for a successful pulmonary vein isolation: acute outcome and follow up from a large multicenter Italian clinical setting | Schiavone, M. et al 202427 Early Clinical Outcomes and Advantages of a Novel-Size Adjustable Second-Generation Cryoballoon: A Proof-of-Concept Study. |
| Iacopino, et al., 2022⁹ Key characteristics for effective acute pulmonary vein isolation when using a novel cryoballoon technology: insights from the CHARISMA registry | Liao Y., et al., 202428 Quantifying pulmonary vein antrum contact area with novel dual-sized cryoballoon to optimize pulmonary vein isolation |
| Martin, et al., 2022¹⁰ Novel cryoballoon to isolate pulmonary veins in patients with paroxysmal atrial fibrillation: long‑term outcomes in a multicentre clinical study | van Boven N., et al., 202529 Prospective evaluation of antral lesion size of the 31-mm size of a novel size-adjustable cryoballoon: Results of the BETTER-FIT study |

| Procedural characteristics | PLX | AFA PRO | p-value |
| Mean minimal cryoballoon temp | -56.1 ± 8.3°C | -46.9 ± 10.1°C | < 0.0001 |
| Real-time visualization of PVI | 71% of PVs | 46% of PVs | < 0.001 |
| Procedure time | 54.5 ± 17.1 min | 59.4 ± 18.6 min | 0.0509 |
| Periprocedural Major or Minor complications | No difference | ||
| Variables | PLX | AFA Pro | p-value |
| Procedure duration, minutes | 60.50 ± 14.23 | 73.43 ± 13.26 | 0.001 |
| Fluoroscopy duration, minutes | 12.83 ± 6.03 | 17.23 ± 7.17 | 0.01 |
| Contrast used, mL | 62.17 ± 7.84 | 60.17 ± 8.03 | 0.9 |
| Number of occlusion | 1.09 ± 0.3 | 1.19 ± 0.5 | 0.6 |
| Time to -40ºC, seconds | 30.43 ± 12.53 | 47.96 ± 16.91 | < 0.001 |
| Nadir temperature, ºC | -58.13 ± 6.26 | -49.63 ± 6.19 | < 0.001 |
| Real time isolation | 101 (84%) | 84 (70%) | 0.009 |
| Isolation time, seconds | 34.47 ± 21.23 | 34.18 ± 26.79 | 0.9 |
| Isolation temperature, ºC | -35.5 ± 13.36 | -29.58 ± 11.27 | 0.002 |
| Thaw time to 0ºC, seconds | 19.31 ± 7.9 | 10.0 ± 4.13 | < 0.001 |
| Acute procedural complications | PLX | AFA Pro | p-value |
| Transient right-sided phrenic nerve palsy | 3.0% | 3.0% | 1.000 |
| Multi-center | Single-center |
| Yap, et al., 2022¹⁵ Comparison of the 1‑year clinical outcome of a novel cryoballoon to an established cryoballoon | Badertscher et al., 2023²⁰ Efficacy and safety of pulmonary vein isolation with pulsed field ablation vs. novel cryoballoon ablation system for atrial fibrillation |
| Knecht, et al., 2022¹⁵ Efficacy and safety of a novel cryoballoon ablation system: multicentre comparison of 1-year outcome | Imnadze, et al., 2022²¹ Two competing cryoballoon technologies for single shot pulmonary vein isolation: first experiences with the novel system |
| Honarbakhsh, et al., 2022¹⁷ POLARx Cryoballoon metrics predicting successful pulmonary vein isolation: targets for ablation of atrial fibrillation | Tilz, et al., 2021²² Novel Cryoballoon Ablation System for Single Shot Pulmonary Vein Isolation – The Prospective ICE-AGE-X Study |
| Guckel, et al., 2022¹⁸ Impact of pulmonary vein variant anatomy and cross‑sectional orifice area on freedom from atrial fibrillation recurrence after cryothermal single‑shot guided pulmonary vein isolation¹⁸ | Creta, et al., 2021²³ First experience of POLARx™ versus Arctic Front Advance™: An early technology comparison |
| Yap, et al., 2021¹⁹ Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: Insights from an initial multicenter experience | Kochi et al., 2021²⁴ Cryoballoon atrial fibrillation ablation: Single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform |
| Pannone L., et al., 202530 Cryoballoon Ablation with the POLARx FIT or the Arctic Front Advance Pro for Paroxysmal Atrial Fibrillation: A Health Economic Analysis |

* mbase, MEDLINE, Web of Science, Cochrane, and Google Scholar databases were searched until 12/01/2022 for studies comparing PLX vs. AFA in PVI patients
| Multi-center | |
| Assaf, Yap et al²⁶ Comparison of procedural efficacy, balloon nadir temperature, and incidence of phrenic nerve palsy between two cryoballoon technologies for pulmonary vein isolation: A systematic review and meta‐analysis |

References
1. Ellenbogen KA et al, One-year outcomes of pulmonary vein isolation with a novel cryoballoon: Primary results of the FROZEN AF trial. J Cardiovasc Electrophysiol. 2024 Apr;35(4):832-842. doi: 10.1111/jce.16220. Epub 2024 Mar 6. PMID: 38448797.
(https://doi.org/10.1111/jce.16220)
2. Su et al. Clinial Application of a Novel 31mm Cryoballoon for Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation: Procedural Data from the FIT arm of FROzEN-AF. HRS 2023
3. Martin et al., Biophysical parameters and time to isolation of pulmonary veins with a novel cryoballoon: results of POLAR ICE study, EP Europace, Volume 24, Issue Supplement_1, May 2022, euac053.078
(https://doi.org/10.1093/europace/euac053.078)
4. Martin CA, Tilz RRR, Anic A, et al. Acute procedural efficacy and safety of a novel cryoballoon for the treatment of paroxysmal atrial fibrillation: Results from the POLAR ICE study. J Cardiovasc Electrophysiol. 2023 Apr;34(4):833-840. Epub 2023 Feb 23. PMID: 36786515.
(https://doi.org/10.1111/jce.15861)
5. Luik A, Anic A, Asmundis C, et al. Long-term success rates of a stable, low pressure cryoballoon for the treatment of paroxysmal atrial fibrillation: Results of the prospective, international, multicenter POLAR-ICE Study. Presented at: 2023 ESC Congress, Aug. 25-28, 2023; Amsterdam, Netherlands.
6. Heeger CH, Pott A, Sohns C, et al. Novel cryoballoon ablation system for pulmonary vein isolation: multicenter assessment of efficacy and safety-ANTARCTICA study. Europace. 2022 Dec 9;24(12):1917-25
(https://doi.org/10.1093/europace/euac148)
7. Spera F et al. Ultra-high-resolution assessment of lesion extension after cryoballoon ablation for pulmonary vein isolation. Front. Cardiovasc. Med. 9:985182. 2022
(https://doi.org/10.3389/fcvm.2022.985182)
8. Fassini, et al., Novel cryoballoon technology for a successful pulmonary vein isolation: acute outcome and follow up from a large multicenter Italian clinical setting, Europace 2022,
(https://doi.org/10.1093/europace/euac053.217)
9. Iacopino, et al., Key characteristics for effective acute pulmonary vein isolation when using a novel cryoballoon technology: insights from the CHARISMA registry, J Interv Cardiovasc Electrophysiol. 2022,
(https://doi.org/10.1007/s10840-021-01063-2)
10. Martin A, et al., Novel cryoballoon to isolate pulmonary veins in patients with paroxysmal atrial fibrillation: long‑term outcomes in a multicentre clinical study, J Interv Cardiovasc Electrophysiol. 2022,
(https://doi.org/10.1007/s10840-022-01200-5)
11. Anic, et al., Acute safety, efficacy, and advantages of a novel cryoballoon ablation system for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: initial clinical experience, Europace 2021,
(https://doi.org/10.1093/europace/euab018)
12. Heeger C-H, Popescu SS, Inderhees T. et al. Novel or established cryoballoon ablation system for pulmonary vein isolation: the prospective ICE-AGE-1 study. Europace. 2023 Aug 2;25(9):euad248.
(https://doi.org/10.1093/europace/euad248)
13. Honarbakhsh S et al., Atrial fibrillation cryoablation is an effective day case treatment: the UK PolarX vs. Arctic Front Advance experience. EP Europace, Volume 25, Issue 11, November 2023, euad286,
(https://doi.org/10.1093/europace/euad286)
14. Mojica J, Lipartiti F, Al Housari M, et al. Procedural safety and efficacy for pulmonary vein isolation with the novel POLARx™ Cryoablation System: A propensity score matched comparison with the Arctic Front™ Cryoballoon in the setting of paroxysmal atrial fibrillation. J Atr Fibrillation. 2021 Jun 30;14(1):20200455.
(https://doi.org/10.4022/jafib.20200455)
15. Yap, et al., Comparison of the 1‑year clinical outcome of a novel cryoballoon to an established cryoballoon, J Interv Cardiovasc Electrophysiol. 2022
(https://doi.org/10.1007/s10840-022-01262-5)
16. Knecht, et al., Efficacy and safety of a novel cryoballoon ablation system: multicentre comparison of 1-year outcome, Europace 2022,
(https://doi.org/10.1093/europace/euac094)
17. Honarbakhsh, et al., POLARx Cryoballoon metrics predicting successful pulmonary vein isolation: targets for ablation of atrial fibrillation, Europace 2022
(https://doi.org/10.1093/europace/euac100)
18. Guckel, et al., Impact of pulmonary vein variant anatomy and cross‑sectional orifice area on freedom from atrial fibrillation recurrence after cryothermal single‑shot guided pulmonary vein isolation, J Cardiovasc Electrophysiol. 2022
(https://doi.org/10.1007/s10840-022-01279-w)
19. Yap, et al., Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: Insights from an initial multicenter experience, J Cardiovasc Electrophysiol. 2021,
(https://doi.org/10.1111/jce.14915)
20. Badertscher et al., Efficacy and safety of pulmonary vein isolation with pulsed field ablation vs. novel cryoballoon ablation system for atrial fibrillation, Europace (2023) 25, 1–8. 2023 Dec 6;25(12):euad329.
(https://doi.org/10.1093/europace/euad329)
21. Imnadze, et al., Two competing cryoballoon technologies for single shot pulmonary vein isolation: first experiences with the novel system, Rev. Cardiovasc. Med. 2022
(http://doi.org/10.31083/j.rcm2304118)
22. Tilz, et al., Novel Cryoballoon Ablation System for Single Shot Pulmonary Vein Isolation – The Prospective ICE-AGE-X Study, Circ J. 2021,
(https://doi.org/10.1253/circj.cj-21-0094)
23. Creta, et al., First experience of POLARx™ versus Arctic Front Advance™: An early technology comparison, J Cardiovasc Electrophysiol. 2021,
(https://doi.org/10.1111/jce.14951)
24. Kochi et al, Cryoballoon atrial fibrillation ablation: Single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform, J Cardiovasc Electrophysiol. Feb 2021
(https://doi.org/10.1111/jce.14930)
25. Assaf A, Rohit E. Bhagwandien, Tamas Szili-Torok, Sing-Chien Yap. Comparison of the acute outcome of two cryoballoon technologies for pulmonary vein isolation: An updated systematic review and meta-analysis, IJC Heart & Vasculature 42 2022,
(https://doi.org/10.1016/j.ijcha.2022.101115)
26. Assaf A, Rohit E. Bhagwandien, Tamas Szili-Torok, Sing-Chien Yap. Comparison of procedural efficacy, balloon nadir temperature, and incidence of phrenic nerve palsy between two cryoballoon technologies for pulmonary vein isolation: A systematic review and meta‐analysis, J Cardiovasc Electrophysiol. 2021,
(https://doi.org/10.1111/jce.15182)
27. Schiavone, M.; Fassini, G.; Moltrasio, M.; Majocchi, B.; Tundo, F.; Casati, F.; Tondo, C. Early Clinical Outcomes and Advantages of a Novel-Size Adjustable Second-Generation Cryoballoon: A Proof-of-Concept Study. J. Clin. Med. 2024, 13, 1259.
(https://www.mdpi.com/2077-0383/13/5/1259)
28. Liao Y, Bai R, Murthy S, Smith DJ, Ebady R, Shatz DY, Weiss JP, Zawaneh M, Tung R, Su W. Quantifying pulmonary vein antrum contact area with novel dual-sized cryoballoon to optimize pulmonary vein isolation. Heart Rhythm. 2024 Nov;21(11):2360-2361
(https://www.heartrhythmjournal.com/article/S1547-5271(24)02660-2/fulltext)
29. Nick van Boven, Rohit Bhagwandien, Sip A. Wijchers, Mark Hoogendijk, Bakhtawar Khan Mahmoodi, Sing-Chien Yap, Prospective evaluation of antral lesion size of the 31-mm size of a novel size-adjustable cryoballoon: Results of the BETTER-FIT study, Heart Rhythm O2, Volume 6, Issue 4, 2025, Pages 393-401, ISSN 2666-5018.
(https://doi.org/10.1016/j.hroo.2025.01.013)
30. Pannone L, Uffenorde S, Bosworth Smith A, et al. Cryoballoon Ablation With the POLARx FIT or the Arctic Front Advance Pro for Paroxysmal Atrial Fibrillation: A Health Economic Analysis. JHEOR. 2025;12(1):155-161.
(https://doi.org/10.36469/001c.133223)
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.
All trademarks are the property of their respective owners