AGENT™
Paclitaxel Coated-PTCA Ballon Catheter
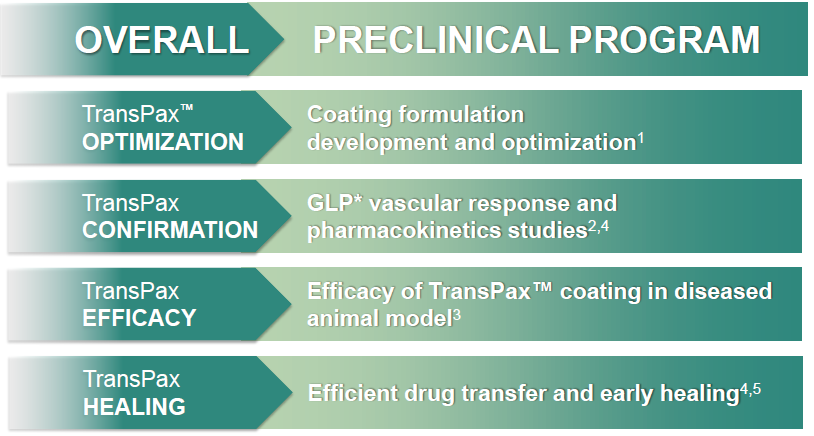
- The safety of AGENT was supported by no device-related morbidity or mortality, no occlusive thrombus, or other adverse clinical events1,2.The vascular response to AGENT treatment groups were comparable to the Pantera™ Lux. No risk to patient safety was identified2,4.
- AGENT achieves similar long term paclitaxel levels with a lower paclitaxel dose density compared to Pantera Lux and SeQuent™ Please1,3.
- Increased drug delivery efficiency allows for a steady state of drug level in the tissue during the restenotic cascade, and for reduced downstream particulates4,5,6.











