AGENT™
Paclitaxel Coated-PTCA Balloon Catheter
The AGENT™ Paclitaxel Drug Coated Balloon catheter, with its innovative TransPax™ coating technology1, provides a targeted, therapeutic dose of proven, anti-proliferative Paclitaxel to the lesion2. It was designed to minimise downstream particulates while maintaining the outstanding deliverability you’ve come to expect with Boston Scientific products3.
Key Resources
Explore
Product Details
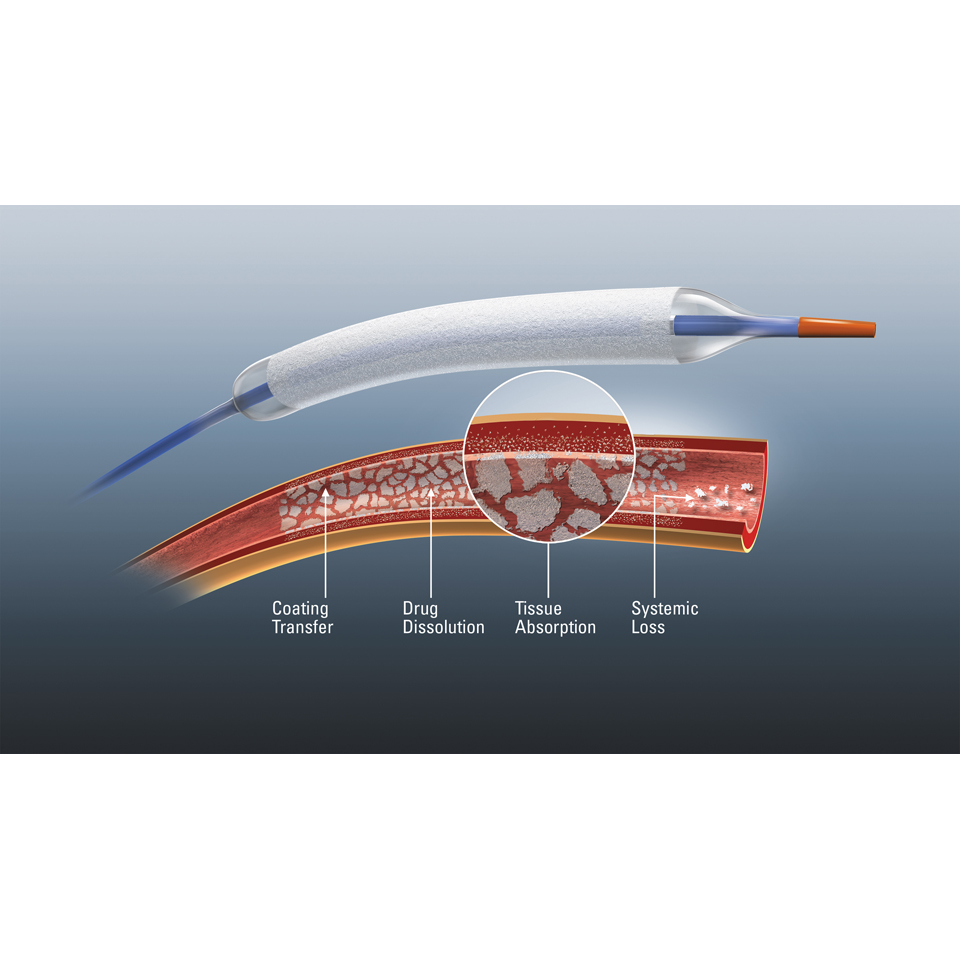
Optimized Drug Transfer
- TransPaxTM coating ensures targeted delivery of Paclitaxel†
- Optimal dose of 2 μg/mm2 compared to 3 μg/mm2 of market leading DCBs‡
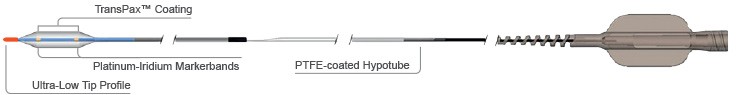
Outstanding Deliverability5
- AgentTM DCB offers exceptional deliverability leveraging the EMERGETM PTCA Catheter:
- Designed to navigate and cross challenging lesions
- Ultra-low tip profile
- Bi-segment inner shaft: flexible distal segment and pushable proximal segment.
Enhanced Coating Integrity
- Balanced hydrophobic and hydrophilic properties of Trans PaxTM coating ensures balloon reaches the lesion with the optimal amount of drug for your patient.
- Fewer particulates are lost distally during the procedure§.
What is TransPax™
The TransPax coating is a proprietary formulation of Paclitaxel and a citrate ester excipient (acetyl tributyl citrate – ATBC). It leverages Boston Scientific’s history and expertise with paclitaxel and drug coating.
Boston Scientific’s TransPax™ is the coating on both IC and PI Drug-coated balloons (DCB). Ranger™ Paclitaxel-Coated PTA Balloon Catheter is Boston Scientific's DCB for Peripheral Interventions.
What is the Delivery System

Agent Infographic
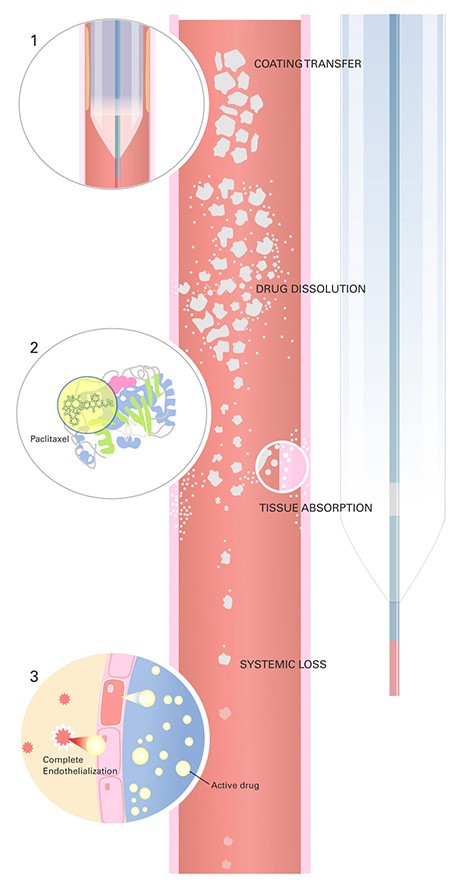
How it works*
1. Ensures that a consistent drug dose is delivered to a lesion*
2. Binds with microtubules to inhibit proliferation of smooth-muscle cells*
3. Enables healing by selectively impacting cells that cause restenosis while allowing healing of endothelial cells*
Safety and performance*
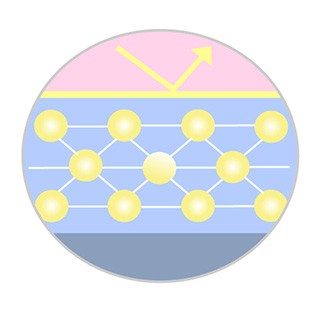
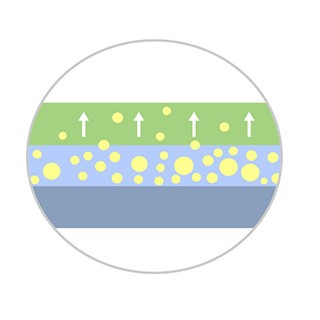
Enhanced Coating Integrity*
Designed to improve coating durability and drug transfer efficacy, allowing for a lower dosage of Paclitaxel*
° Data on file at Boston Scientific. Pre-clinical results may not be necessarily indicative of clinical outcomes.
§ Bench testing performed by Boston Scientific. Results not necessarily indicative of clinical performance.
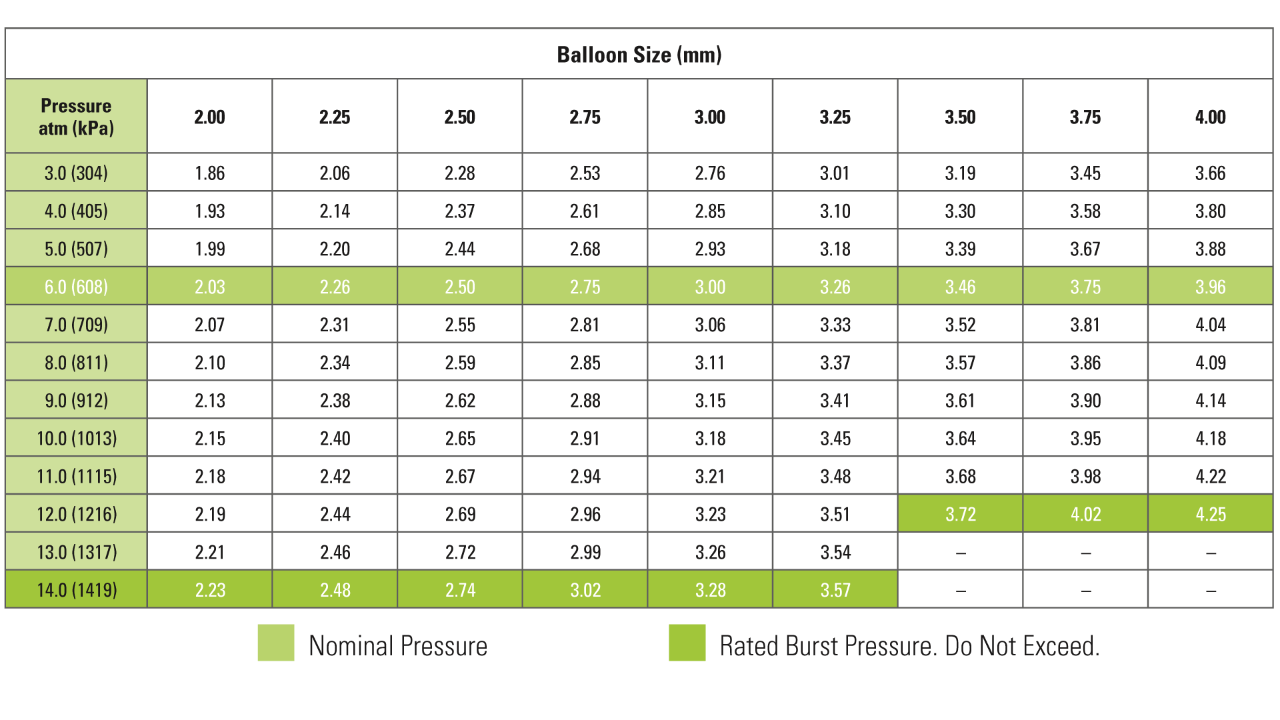
Compliance Chart
Ordering Information
Balloon Length
| Ø(mm) | 12mm | 15mm | 20mm | 30mm |
|---|---|---|---|---|
| 2.00 | H74939222201210 | H74939222201510 | H74939222202010 | H74939222203010 |
| 2.25 | H74939222221210 | H74939222221510 | H74939222222010 | H74939222223010 |
| 2.50 | H74939222251210 | H74939222251510 | H74939222252010 | H74939222253010 |
| 2.75 | H74939222271210 | H74939222271510 | H74939222272010 | H74939222273010 |
| 3.00 | H74939222301210 | H74939222301510 | H74939222302010 | H74939222303010 |
| 3.50 | H74939222351210 | H74939222351510 | H74939222352010 | H74939222353010 |
| 4.00 | H74939222401210 | H74939222401510 | H74939222402010 | H74939222403010 |
† Data on file at Boston Scientific. Pre-clinical results may not be necessarily indicative of clinical outcomes. Hemoteq Coating Process Validation by HPLC. Data on file at Hemoteq.
‡ 3 μg/mm2 dose for both B Braun SeQuent Please and Biotronik Pantera Lux per manufacturer’s website.
§Method: balloons delivered in a coronary track model with fluid recirculation of 150 ml/min at T = 37 ˚C. Particulates are collected with a polycarbonate filter 5.0 μm x 47 mm (Sterilitech).
Bench test results may not necessarily be indicative of clinical performance.