Deliver effective downstaging and bridging treatment

TheraSphereTM Y90 Therapy not only provides you and your patients with time to bridge to future treatments, but can allow downstaging of the tumour to access curative surgery options.
Provide better disease control
➣ >3/4 of HCC patients had a response that lasted more than 6 months following TheraSphere Y90 Therapy as a primary treatment1
Pivotal LEGACY trial1
- Duration of response
- Summary
- Study design
Duration of response >6 months*
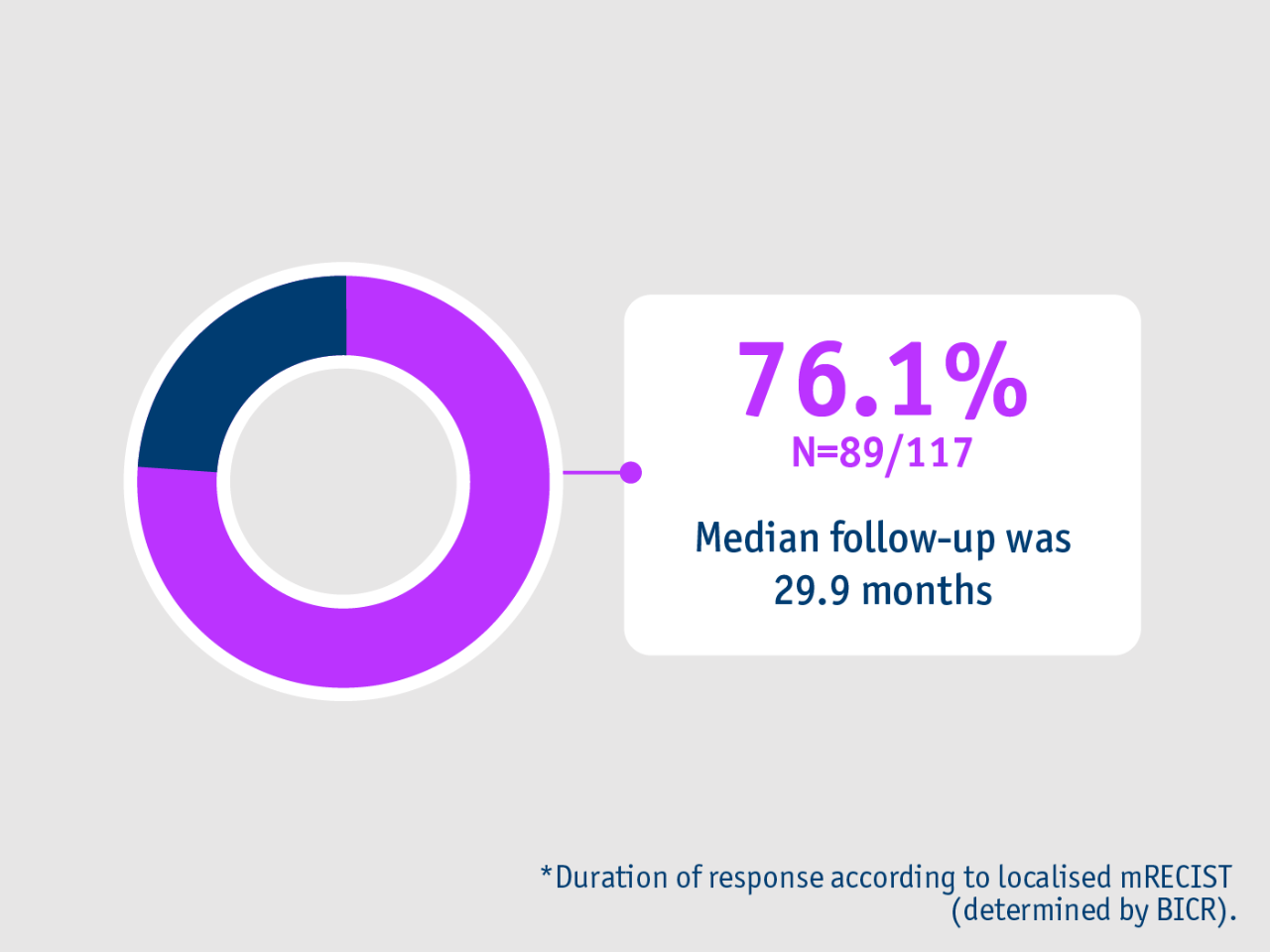
LEGACY trial (Salem et al. 2021)
• Multicentre
• Single-arm
• Retrospective
Patients with solitary unresectable HCC
• Tumour size <8 cm
• Child-Pugh A
• 60% BCLC A, 40% BCLC C
• ECOG 0-1
➣ 72% chance of being free from target lesion progression at Year 5 following TheraSphere Y90 Therapy2
Evidence in early-stage HCC from Lewandowski et al. 20182
- Disease control
- Summary
- Study design
Local tumour control rate at 5 years
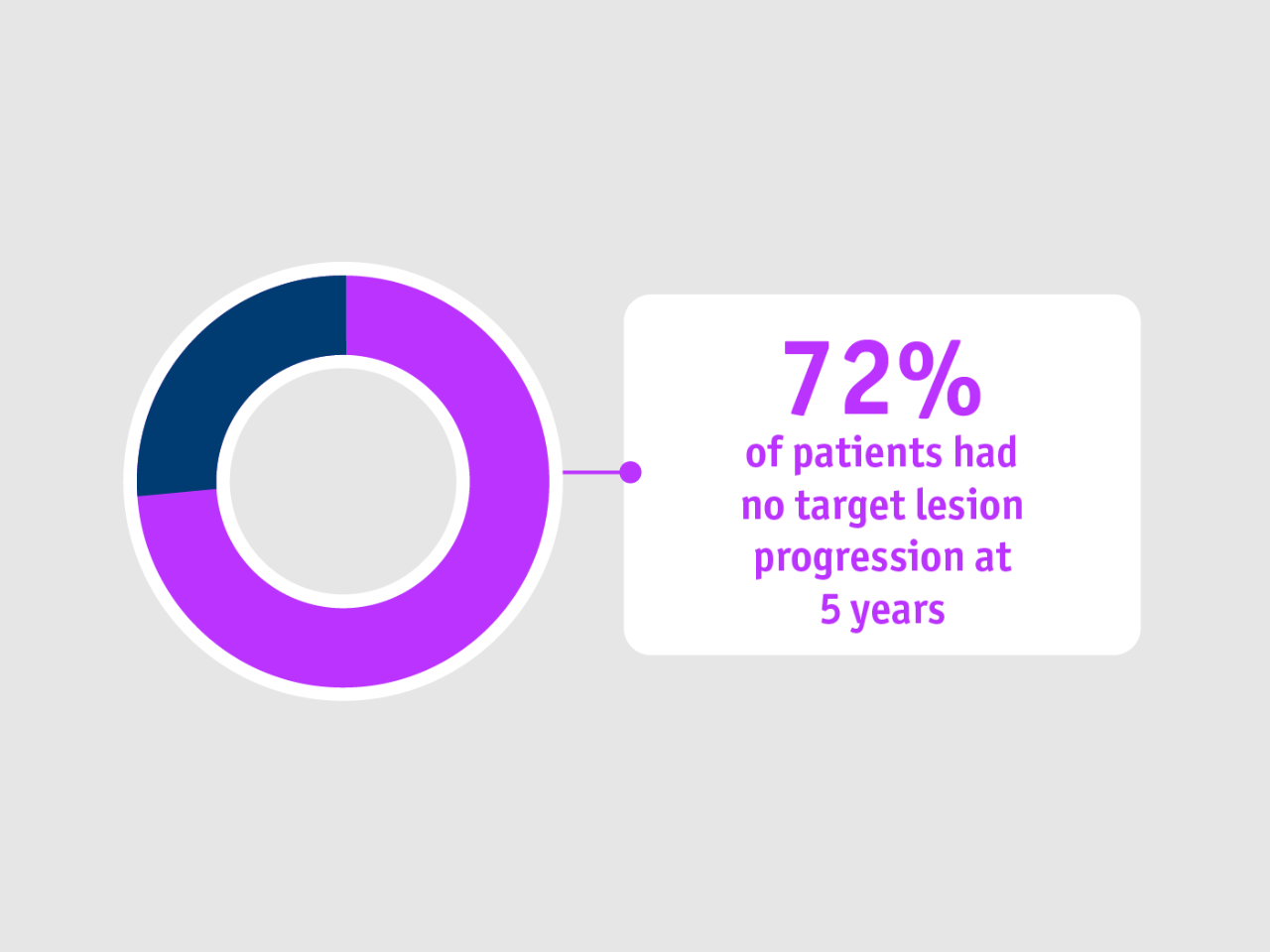
Lewandowski et al. 2018
• Retrospective analysis
• Single-arm
• Single centre (USA)
Patients with solitary HCC not amenable to percutaneous ablation
• BCLC A
• Tumour size ≤5 cm
Promote future liver remnant
➣ All patients with unilobar HCC achieved disease control following neoadjuvant TheraSphere Y90 Therapy3
➣ 77% of tumours showed more than 50% pathologic necrosis at resection3
Longitudinally clinical and radiological outcomes study Gabr et al. 20183
- Future liver remnant
- Clinical outcomes
- Summary
- Study design
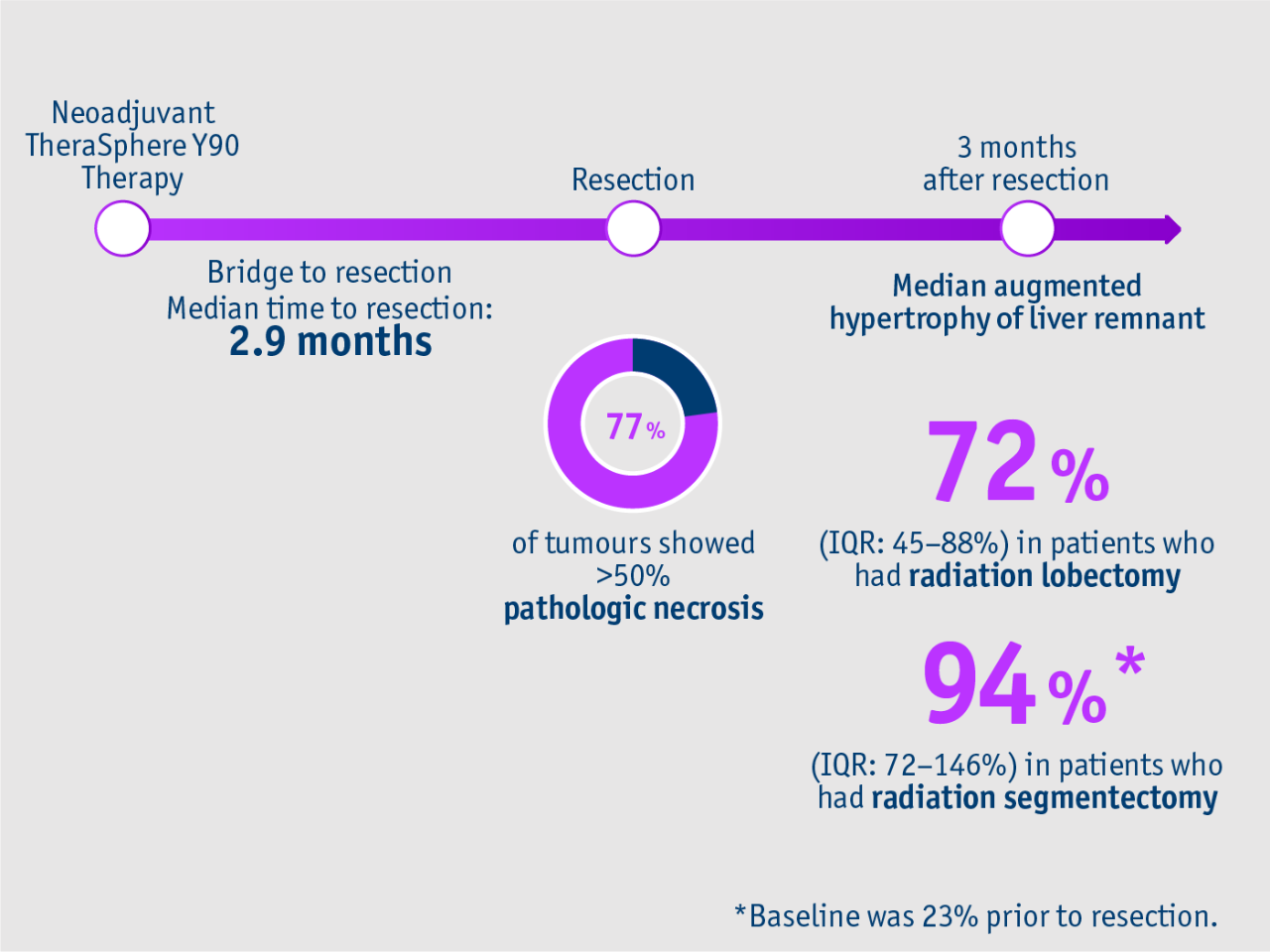
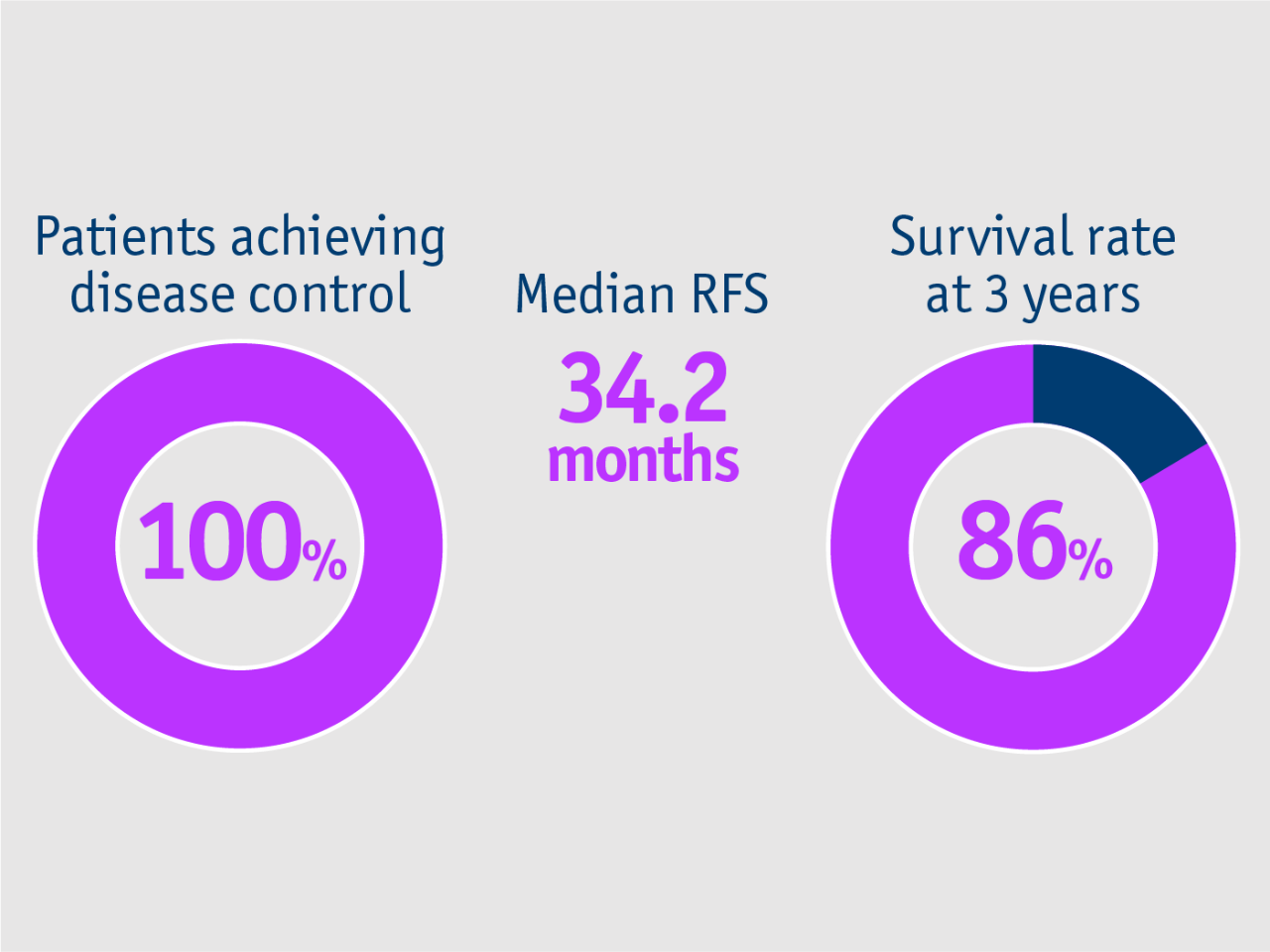
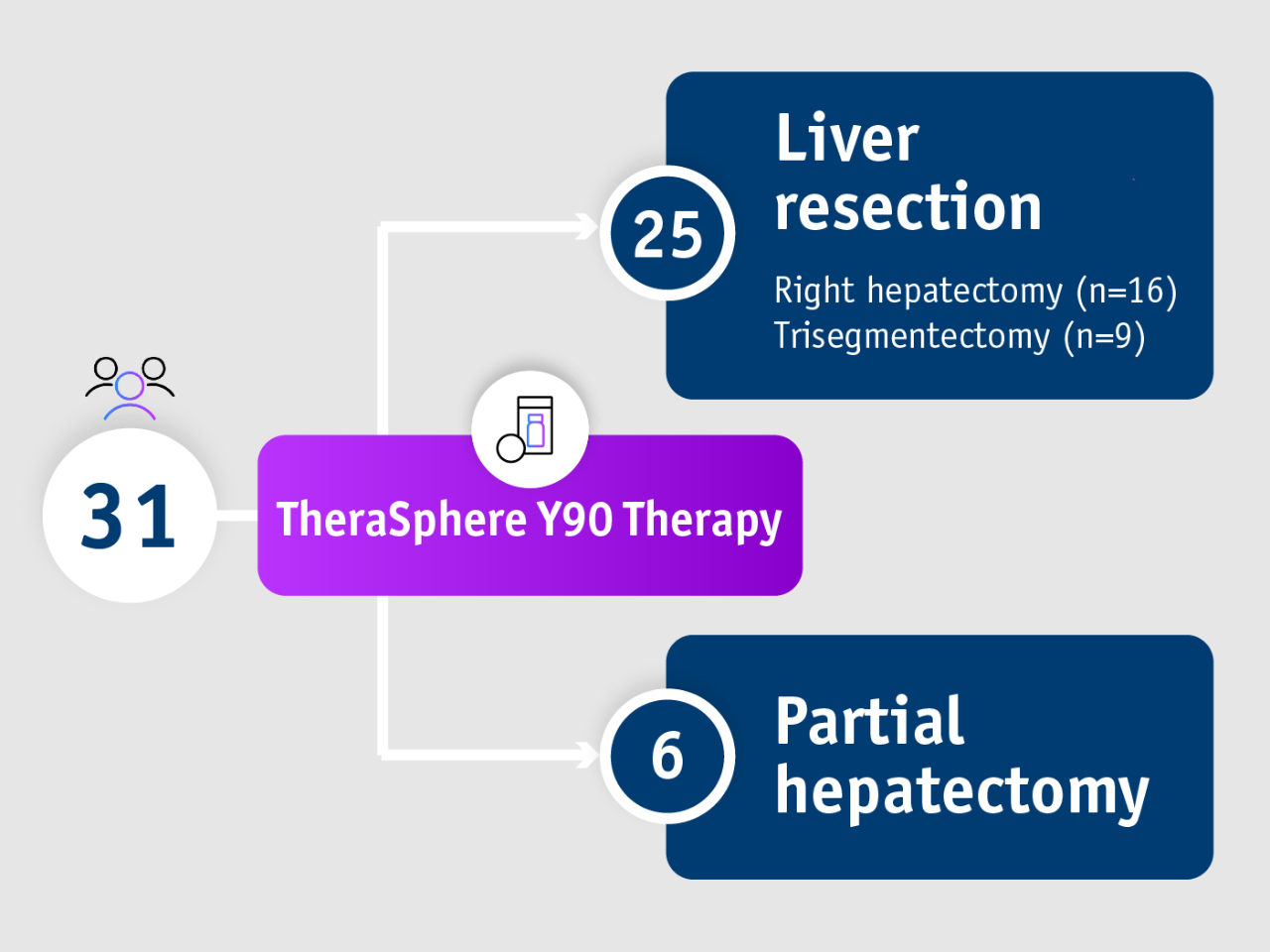
Gabr et al. 2018
• Retrospective
• Single centre (USA)
Patients with unilobar HCC
• Preserved liver function
• Child-Pugh ≤ B7
• No portal vein thrombus or metastatic disease
Effectively downstage more patients
➣ ~2x patients with unresectable HCC
downstaged from stage T3 to T2 following TheraSphere Y90 Therapy vs cTACE4
Lewandowski et al. 20094
- Downstaging
- Tumour reduction
- Summary
- Study design
Proportion of patients downstaged
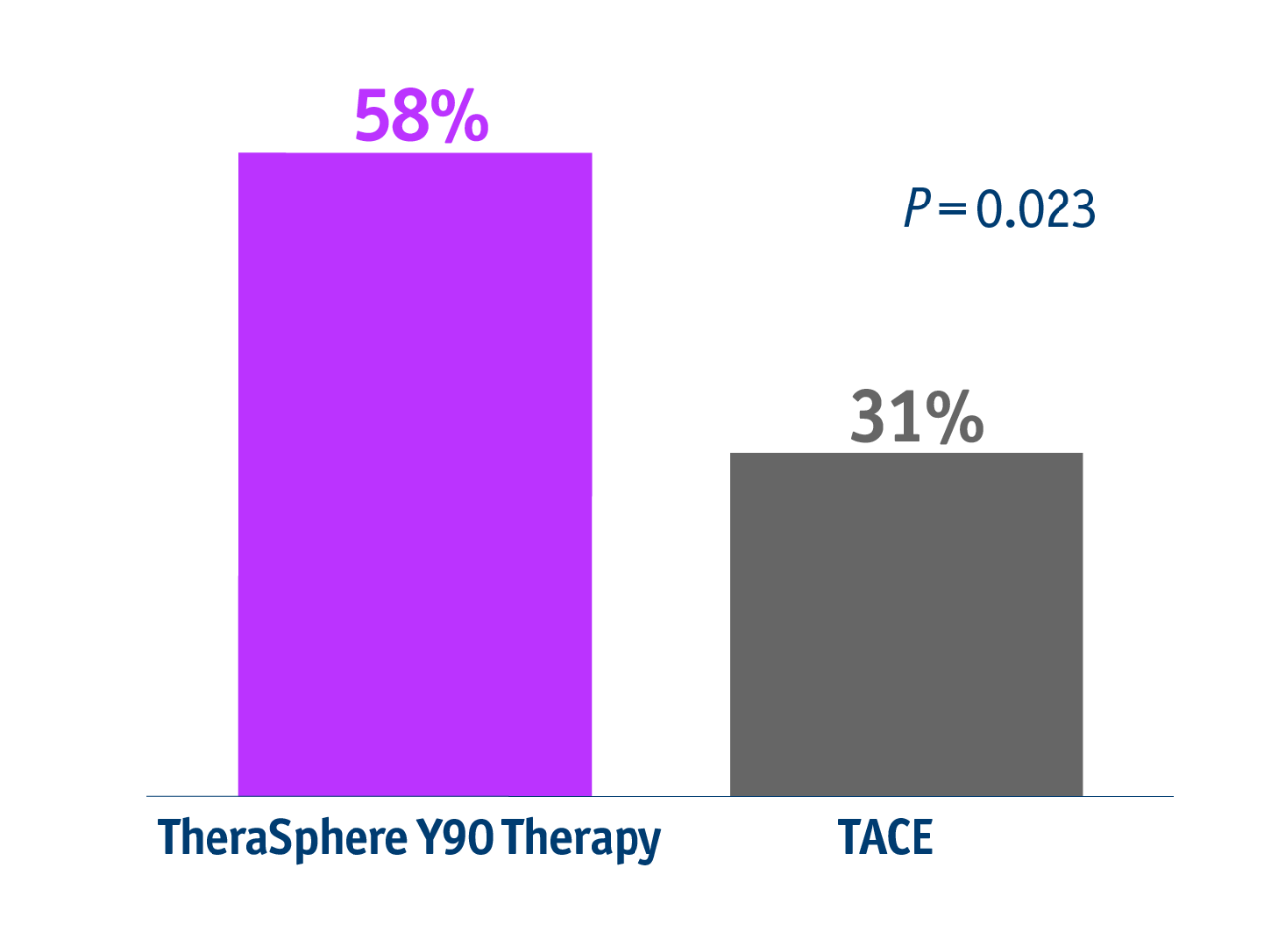
This trend favouring TheraSphere Y90 Therapy for downstaging was maintained for all lesion sizes.
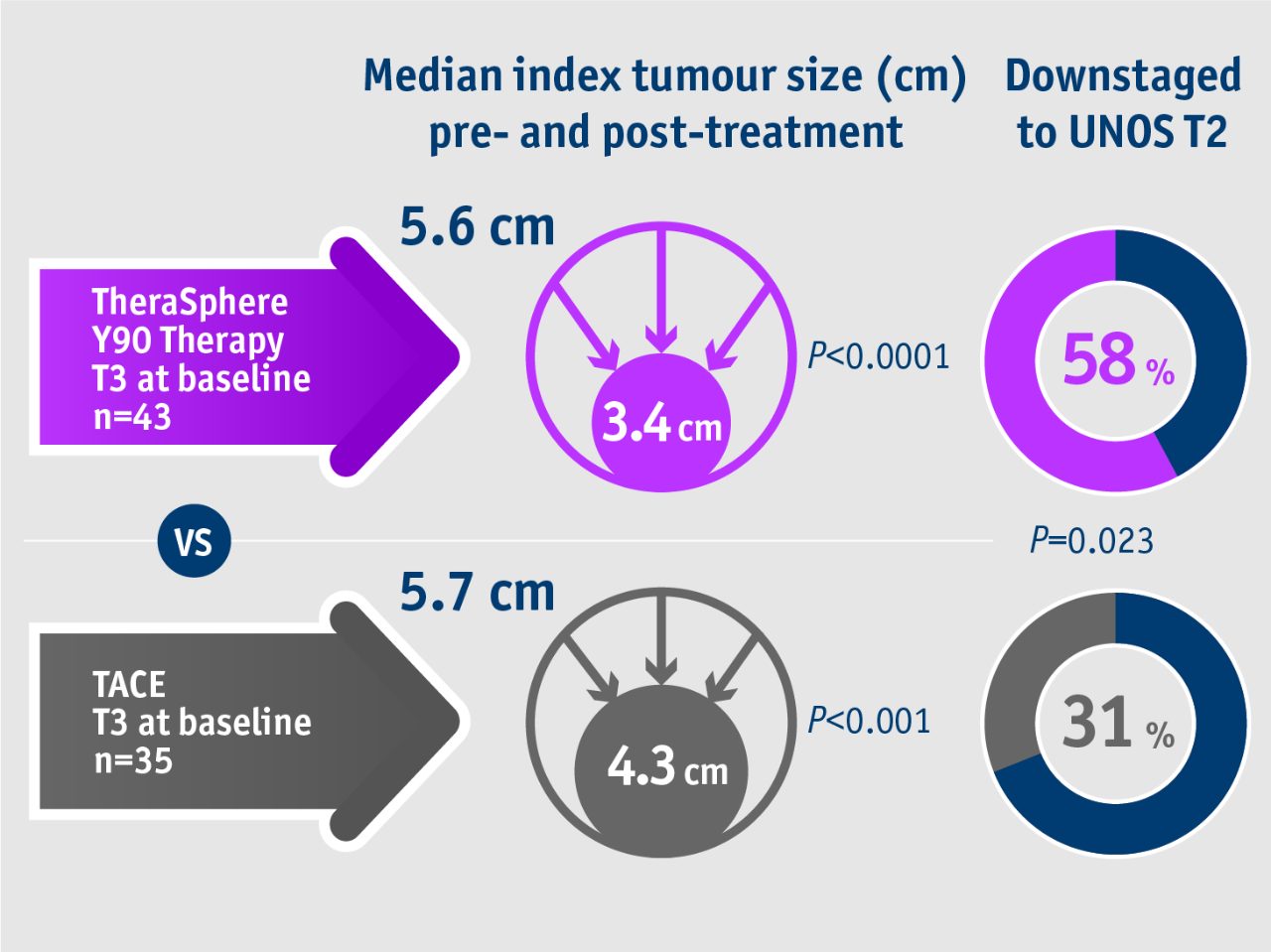
Lewandowski et al. 2009
• Retrospective
• Comparative
• Single centre (USA)
Patients with unresectable HCC
• Stage T3 disease
➣ Long-term data: ~2/3 of patients received liver transplant after downstaging from stage T3 to T2 with TheraSphere Y90 Therapy5
15-year data from Gabr et al. 20215
- Downstaging
- Tumour stage
- Summary
- Study design
Tumour characteristics at TheraSphere Y90 Therapy and transplant
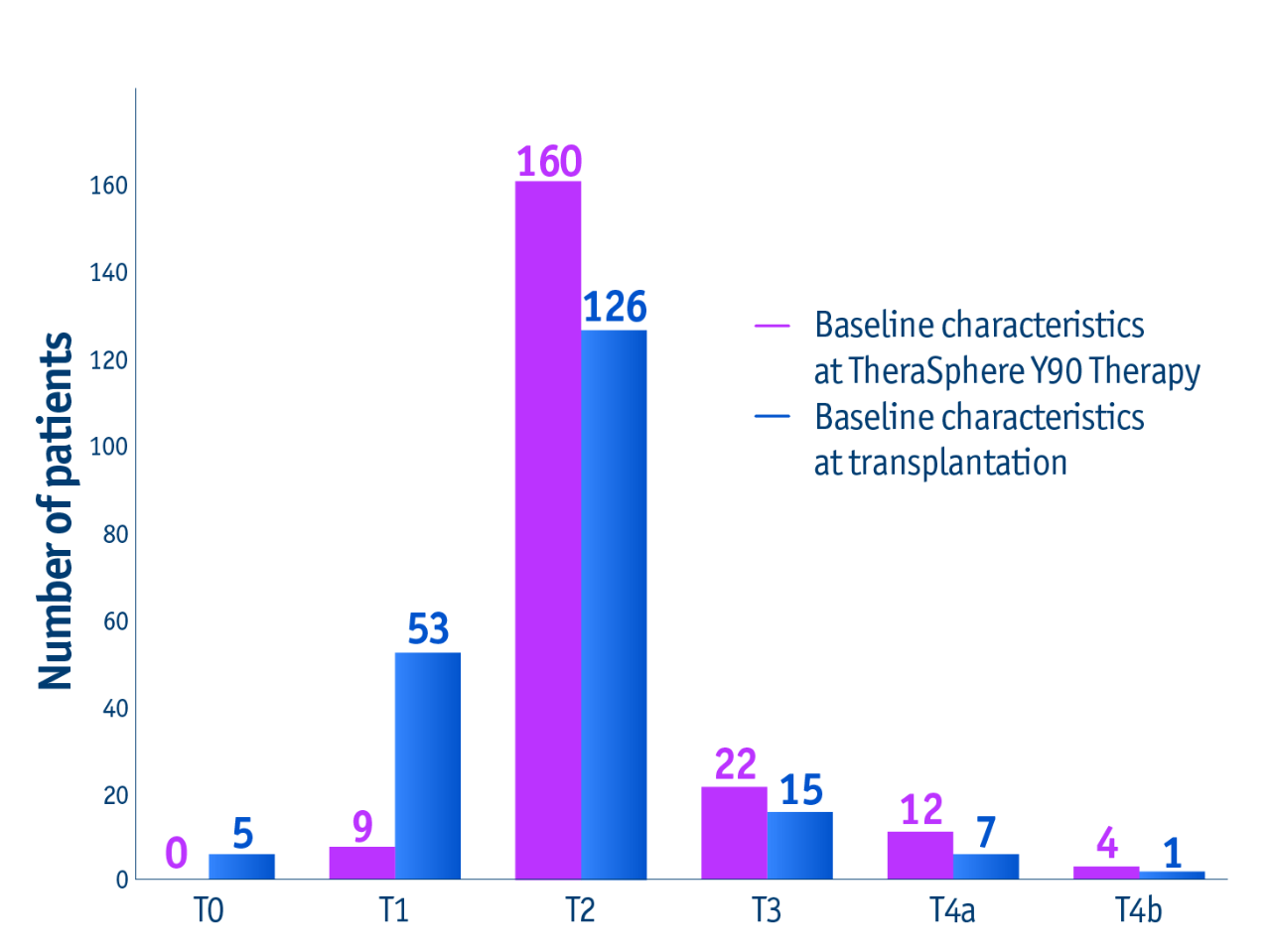
This trend favouring TheraSphere Y90 Therapy for downstaging was maintained for all lesion sizes.
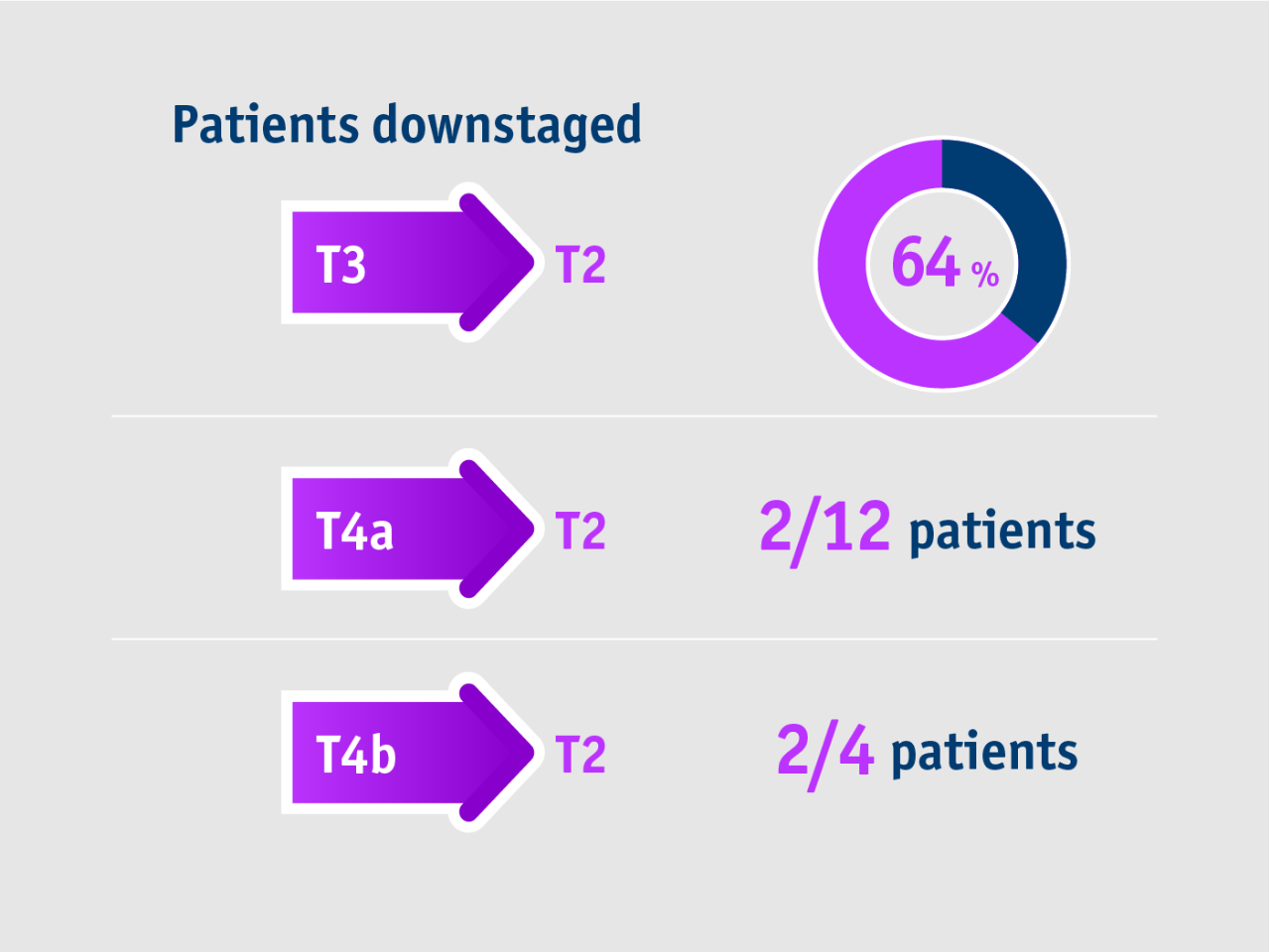
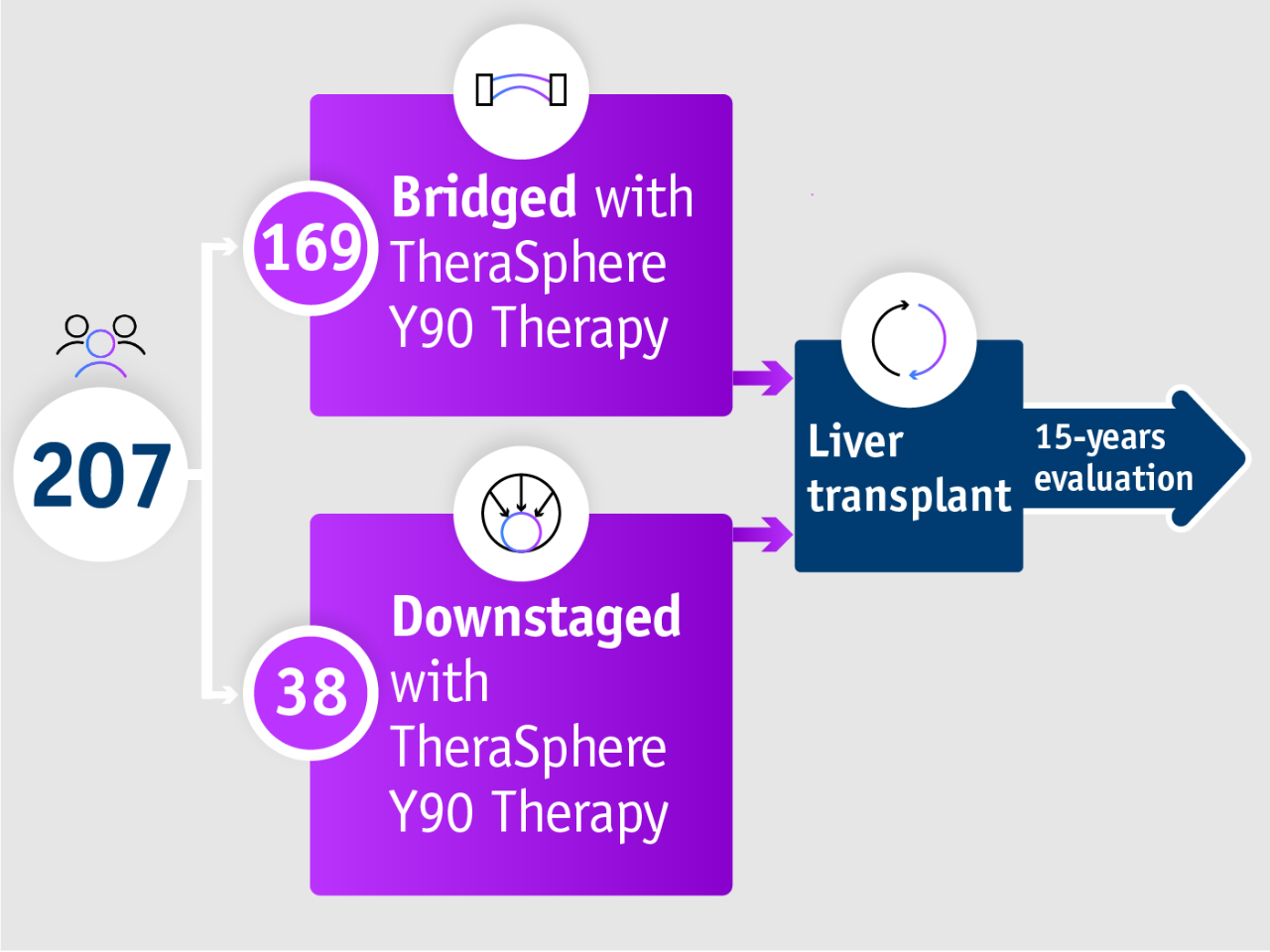
Gabr et al. 2021
• Phase II trial
• Randomised
• Prospective
• Open-label
• Single-centre (USA)
Patients with HCC undergoing liver transplant following downstaging or bridging with TheraSphere Y90 Therapy
• Treatment-naïve (79.5%)
• 51% BCLC A; 31% BCLC C
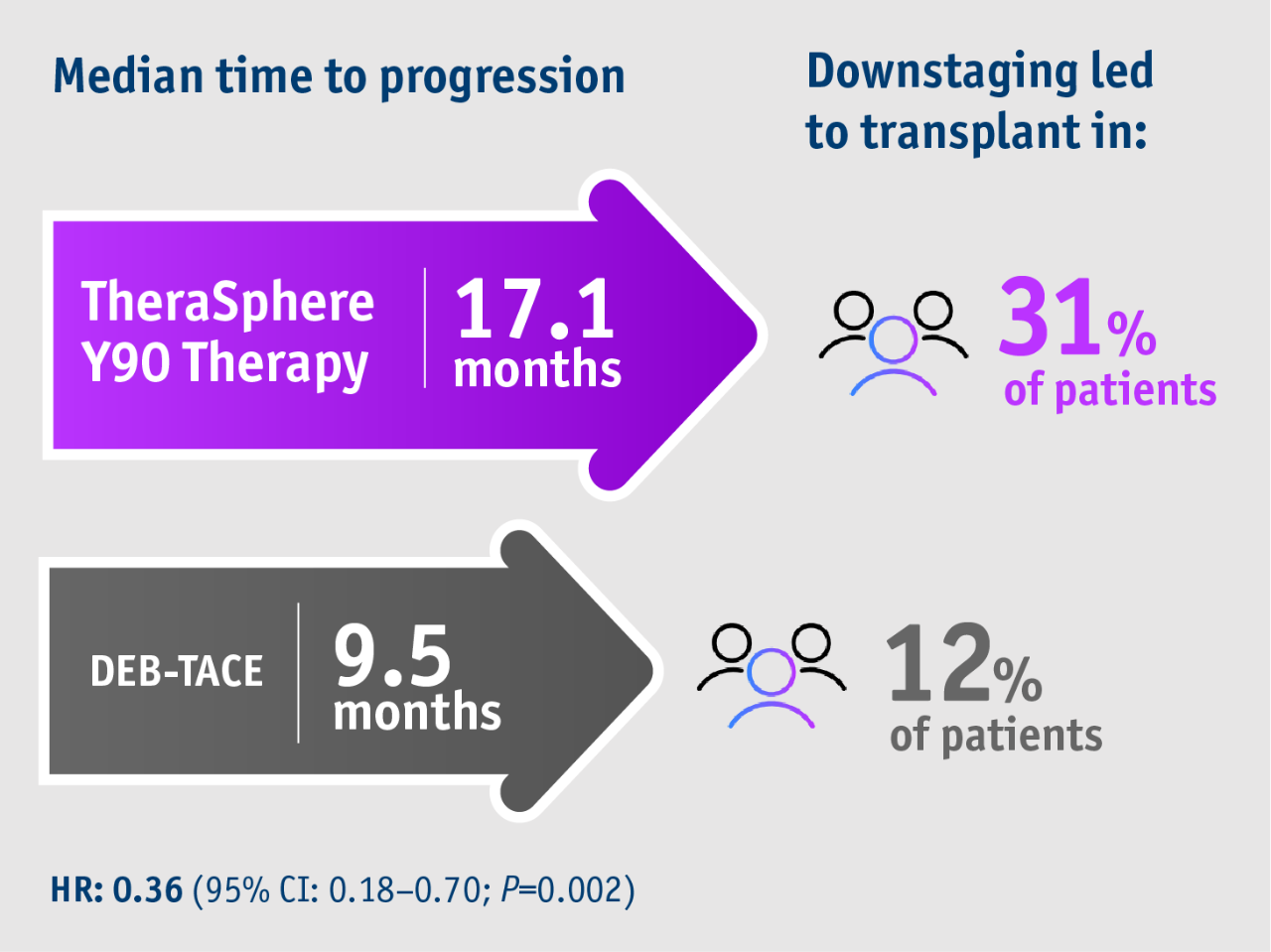
➣ >2x HCC patients initially not amenable to curative treatment downstaged to liver transplant following TheraSphere Y90 Therapy vs DEB-TACE6
Phase II TRACE trial6
- Time to progression
- Downstaging
- Summary
- Study design
Time to overall tumour progression in the ITT group
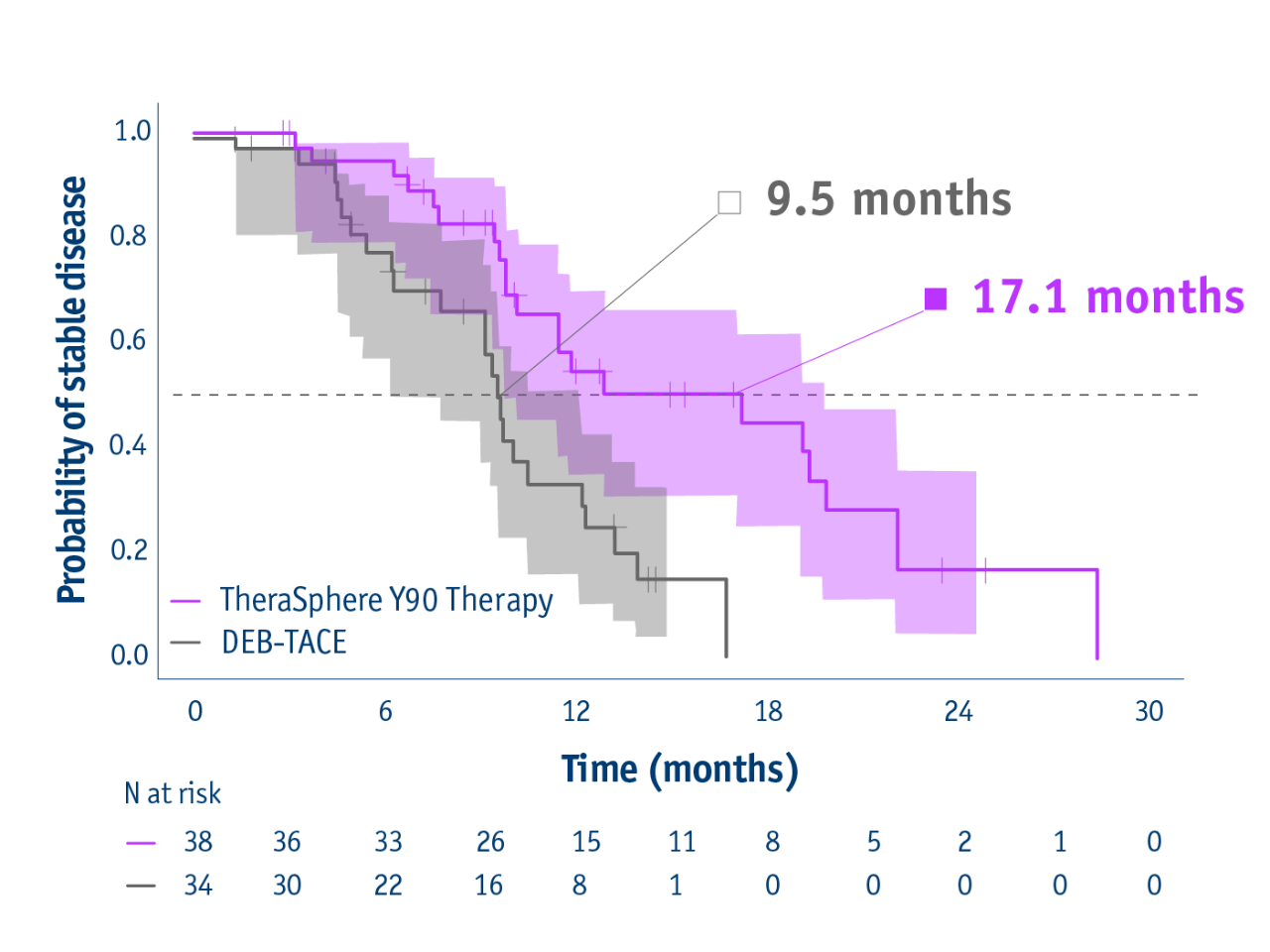
- 17.1 months median TTP with TheraSphere Y90 Therapy
(95% CI: 8.9–25.4) - 9.5 months median TTP with DEB-TACE
(95% CI: 8.8–10.2) - HR: 0.36
(95% CI: 0.18–0.70; P=0.002)
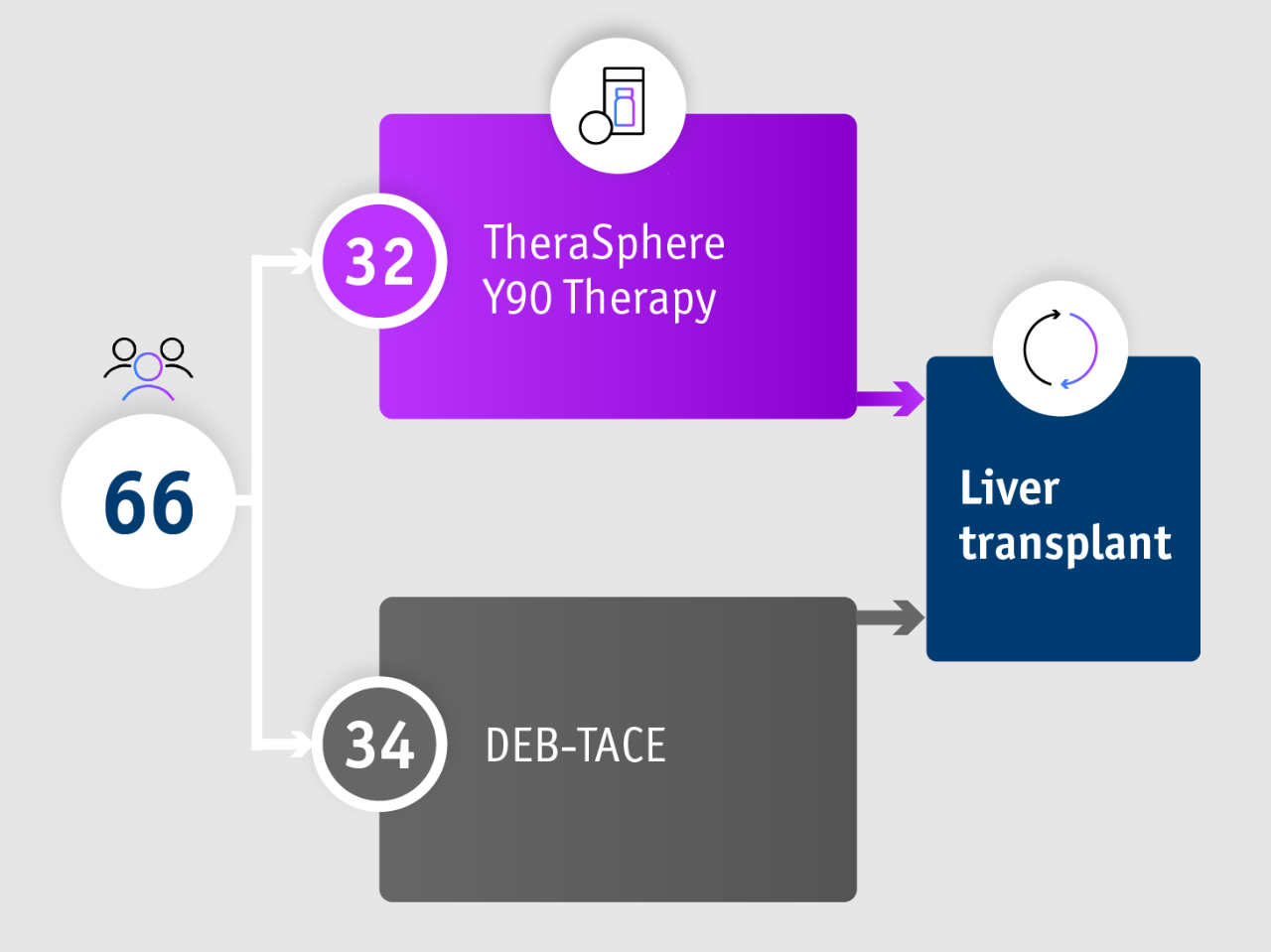
TRACE trial (Dhondt et al. 2022)
• Phase II trial
• Open-label
• Single-centre (Belgium)
• Randomised
• Controlled
Patients with HCC not amenable to curative treatment
• BCLC A/B
• ECOG PS 1
• Segmental PVT
Bridge to gain time
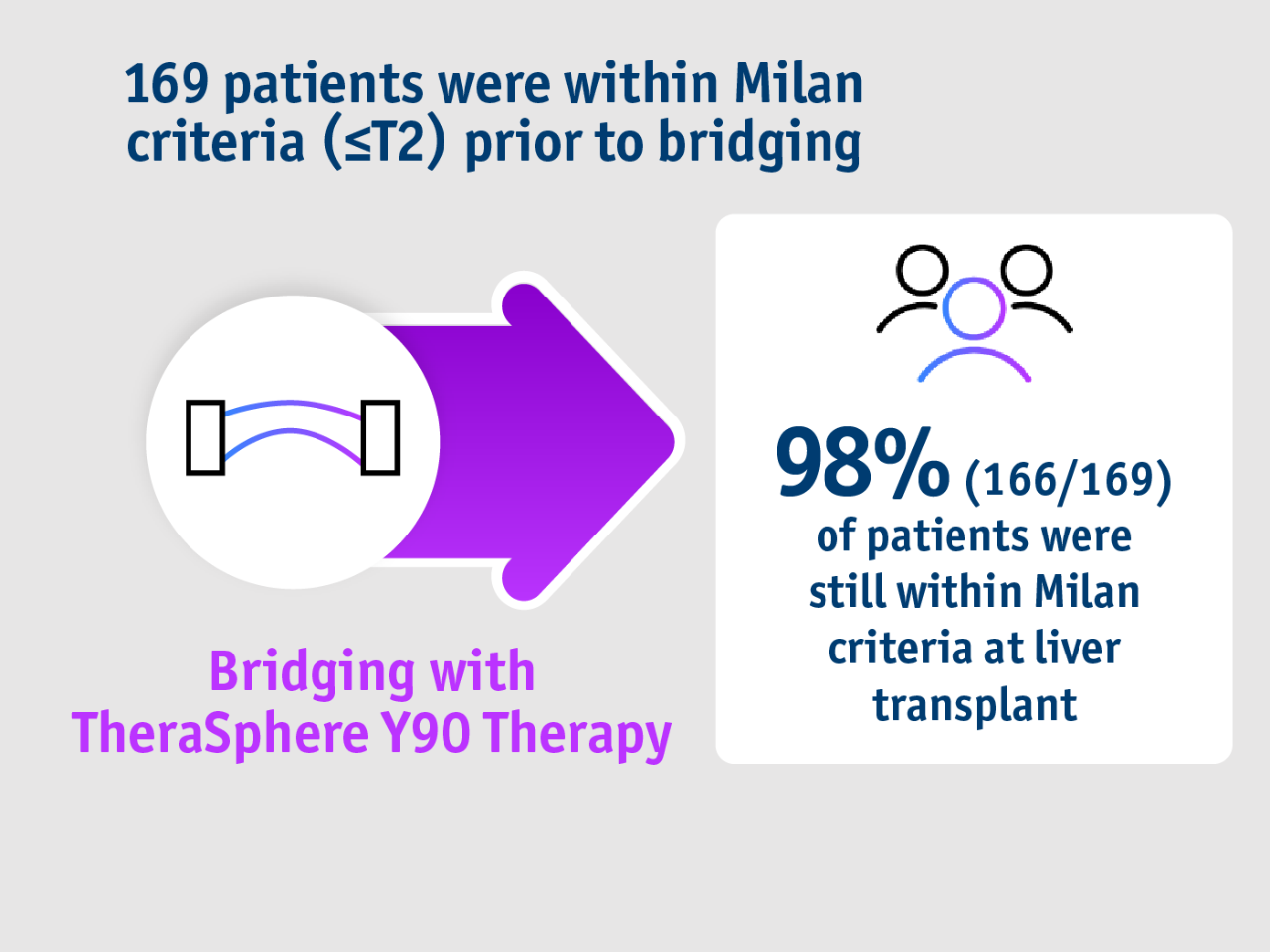
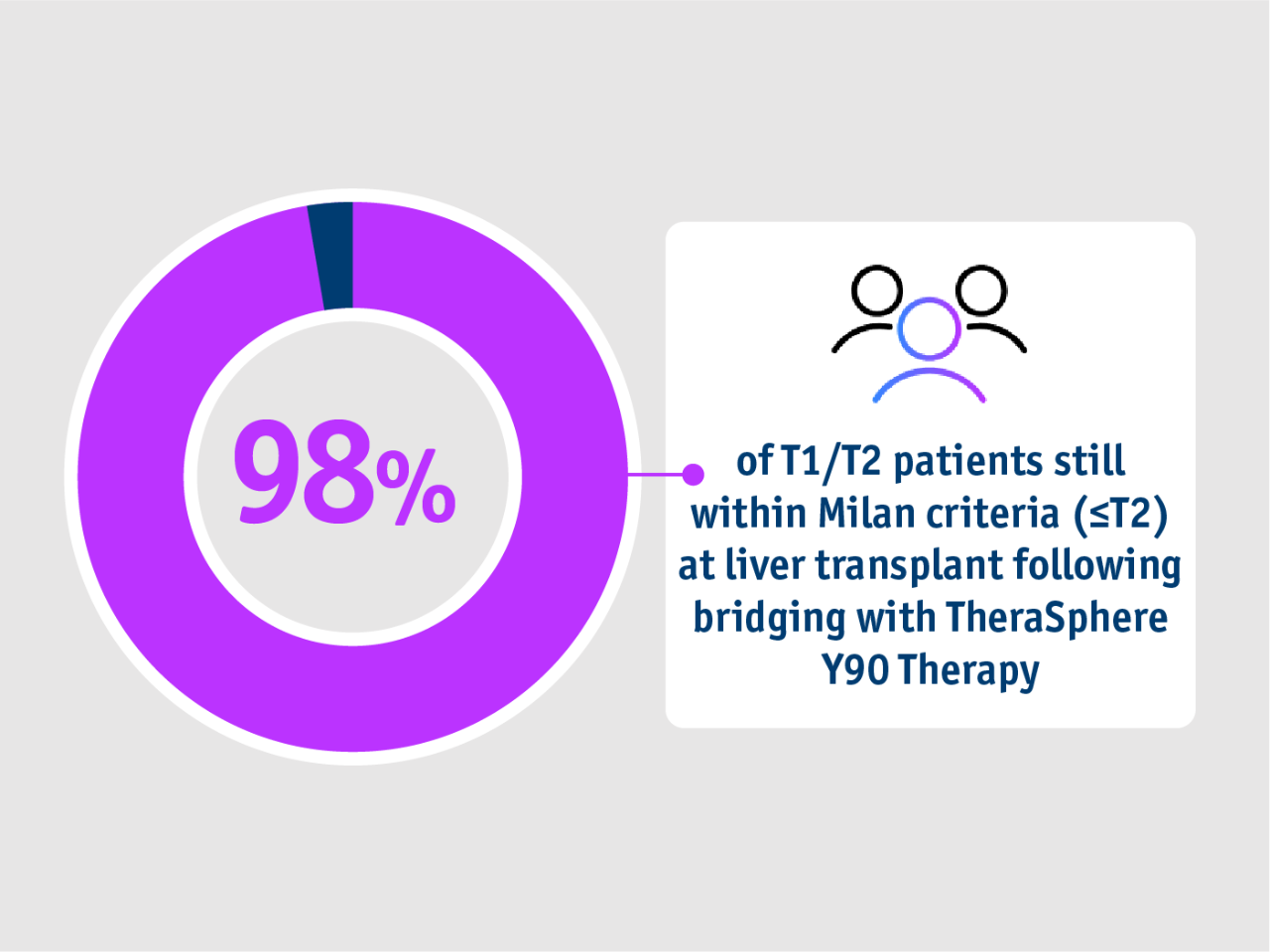
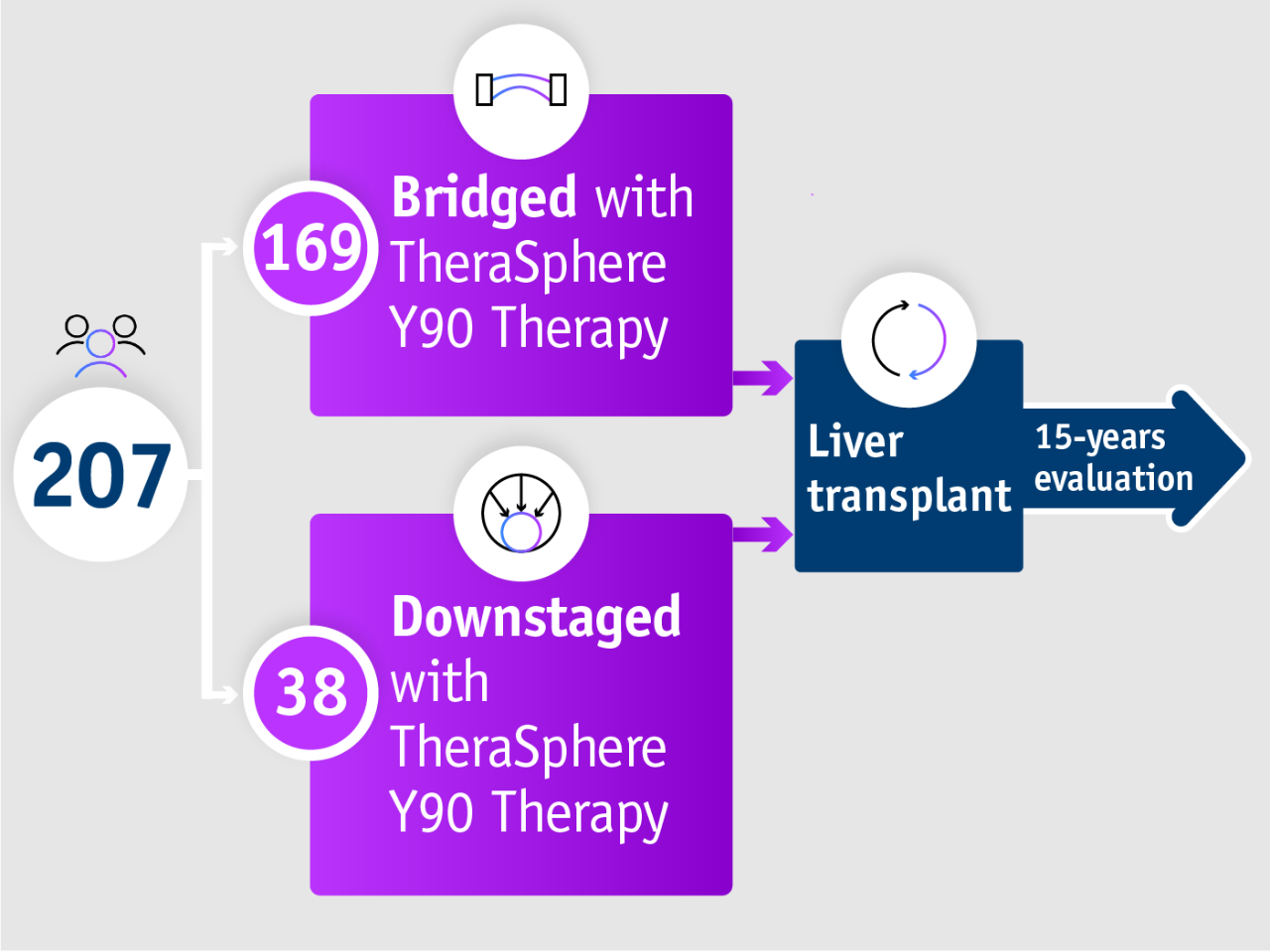
➣ 98% of HCC patients undergoing liver transplant remained within Milan criteria following bridging with TheraSphere Y90 Therapy5
Long term evidence in Gabr et al. 20215
- Bridging
- Milan criteria
- Summary
- Study design
Gabr et al. 2021
• Phase II trial
• Randomised
• Prospective
• Open-label
• Single-centre (USA)
Patients with HCC undergoing liver transplant following downstaging or bridging with TheraSphere Y90 Therapy
• Treatment-naïve (79.5%)
• 51% BCLC A; 31% BCLC C
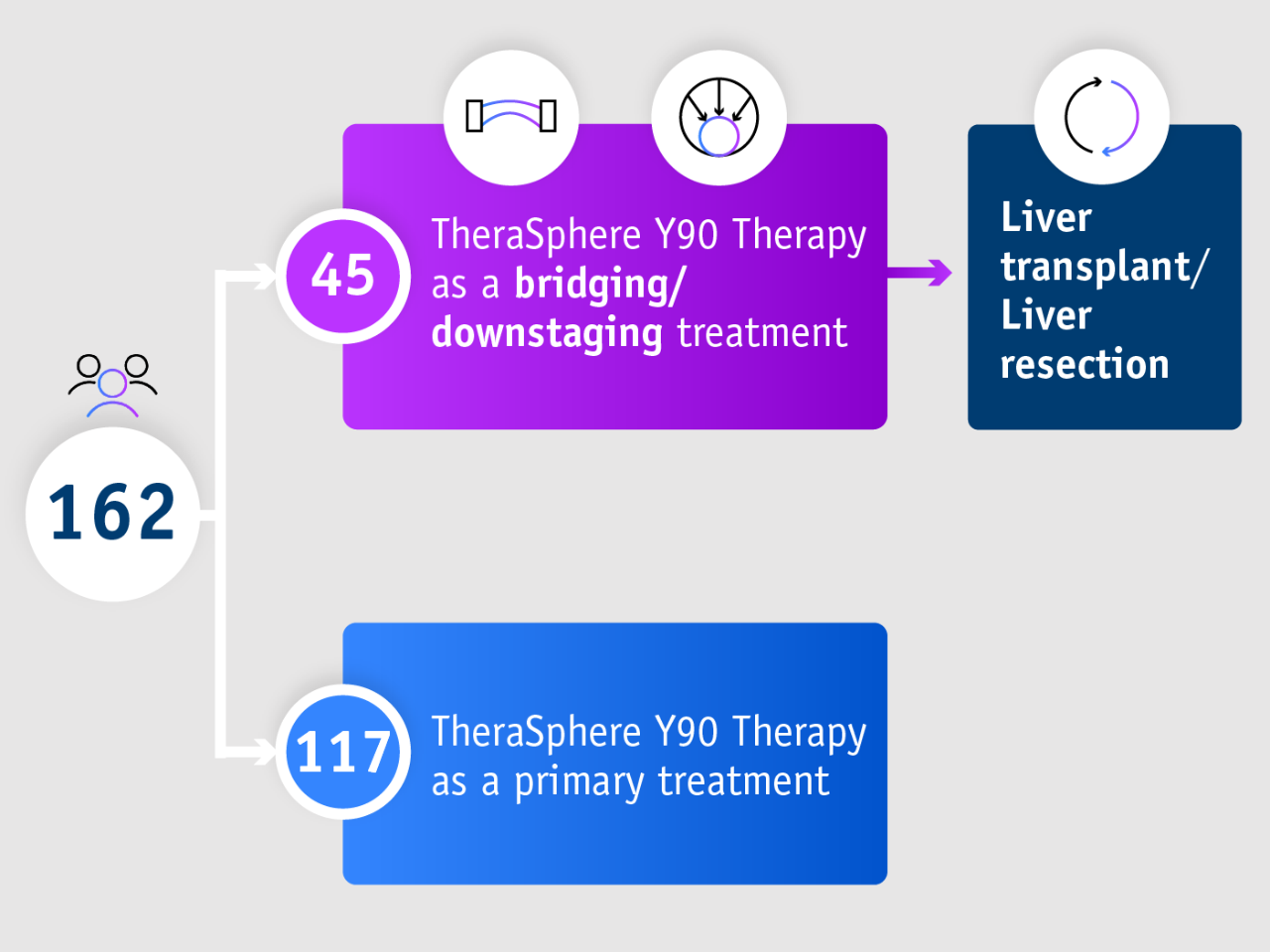
➣ 93% OS rate at 3 years in patients with transplant or resection following TheraSphere Y90 Therapy1
Pivotal LEGACY trial1
- Overall survival
- Summary
- Study design
Overall survival in treated population
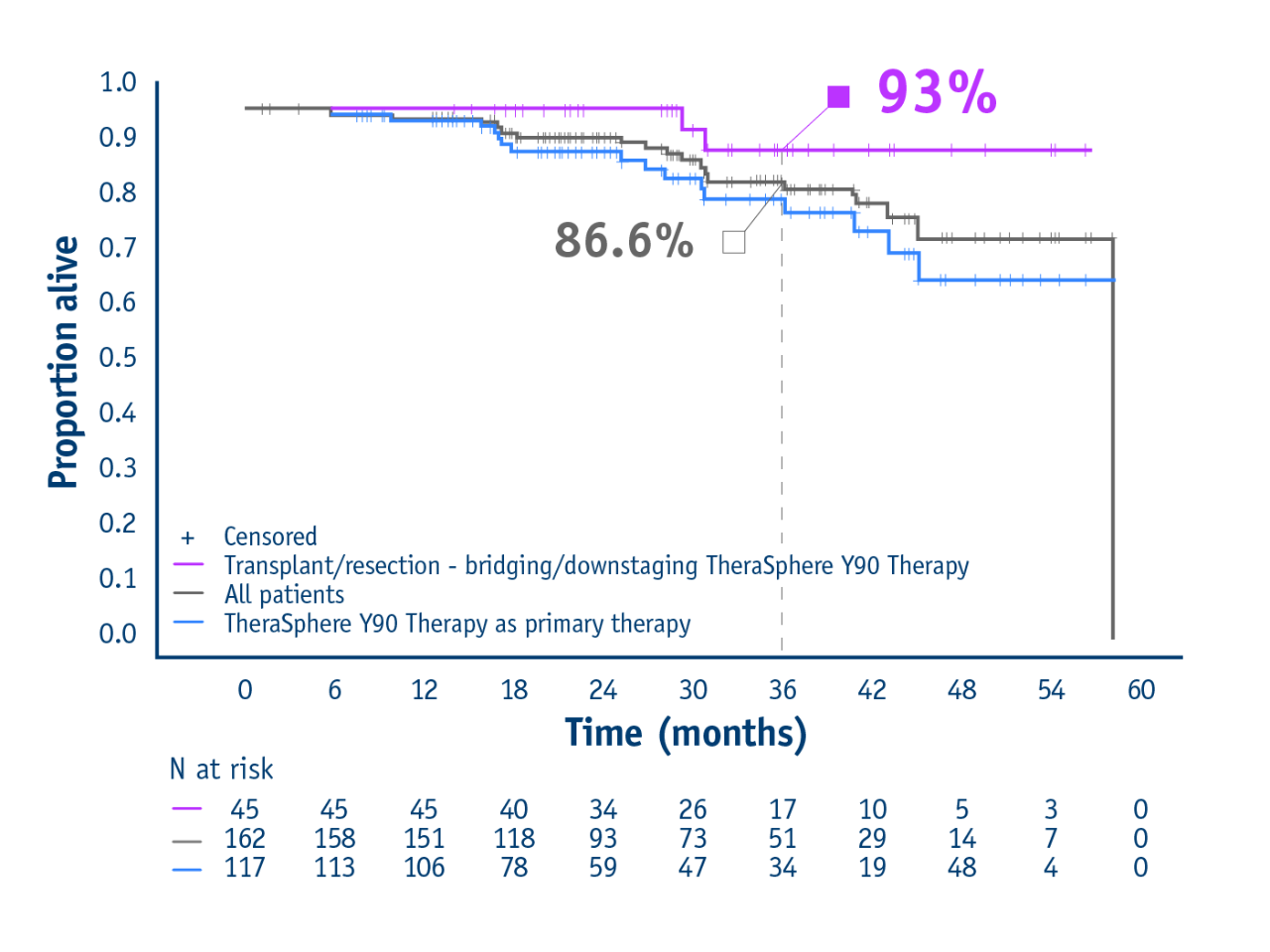
- 93% of patients alive following liver transplant or resection
(95% CI: 74.2–98.2) - 86.6% of all patients alive following TheraSphere Y90 Therapy
(95% CI: 78.2–92.0)
LEGACY trial (Salem et al. 2021)
• Multicentre
• Single-arm
• Retrospective
Patients with solitary unresectable HCC
• Tumour size <8 cm
• Child-Pugh A
• 60% BCLC A, 40% BCLC C
• ECOG 0-1
References
1.Salem R, et al. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021 Nov;74(5):2342-2352.
2.Lewandowski RJ, et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018 Jun;287(3):1050-1058.
3.Gabr A, et al. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018 Nov;29(11):1502-1510.e1.
4.Lewandowski RJ, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009 Aug;9(8):1920-1928.
5.Gabr A, et al. Liver Transplantation Following Yttrium-90 Radioembolization: 15-Year Experience in 207-Patient Cohort. Hepatology. 2021 Mar;73(3):998-1010.
6.Dhondt E, et al. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology. 2022 Jun;303(3):699-710.
Abbreviations
AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; BICR; Blinded independent central review; CI, confidence interval; EASL, European Association for the Study of the Liver; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; ITT, intention to treat; LT, liver transplant; mRECIST, modified Response Evaluation Criteria in Solid Tumors; ORR, objective response rate; OS, overall survival; PVT, portal vein thrombosis; RFS, recurrence-free survival; TACE, transarterial chemoembolisation; TTP, time to progression; UNOS, United Network for Organ Sharing; Y90, yttrium-90.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
















