Preserve healthy tissues with Simplicit90Y personalised dosimetry

Cancer care is multidisciplinary. TheraSphereTM Y90 Therapy harnesses Simplicit90Y personalised dosimetry to be a minimally invasive, well-tolerated interventional treatment for your patients with HCC. It is a proven and precise therapy that expands your capabilities to achieve tumour response while preserving future treatment options.1
Provide greater survival benefits
➣ Doubled overall survival in patients with unresectable locally advanced HCC following personalised TheraSphereTM Y90 Therapy vs standard dosimetry2
DOSISPHERE-01 Level 1 evidence study2
- Overall survival
- Summary
- Study design
Overall survival in the intention-to-treat population
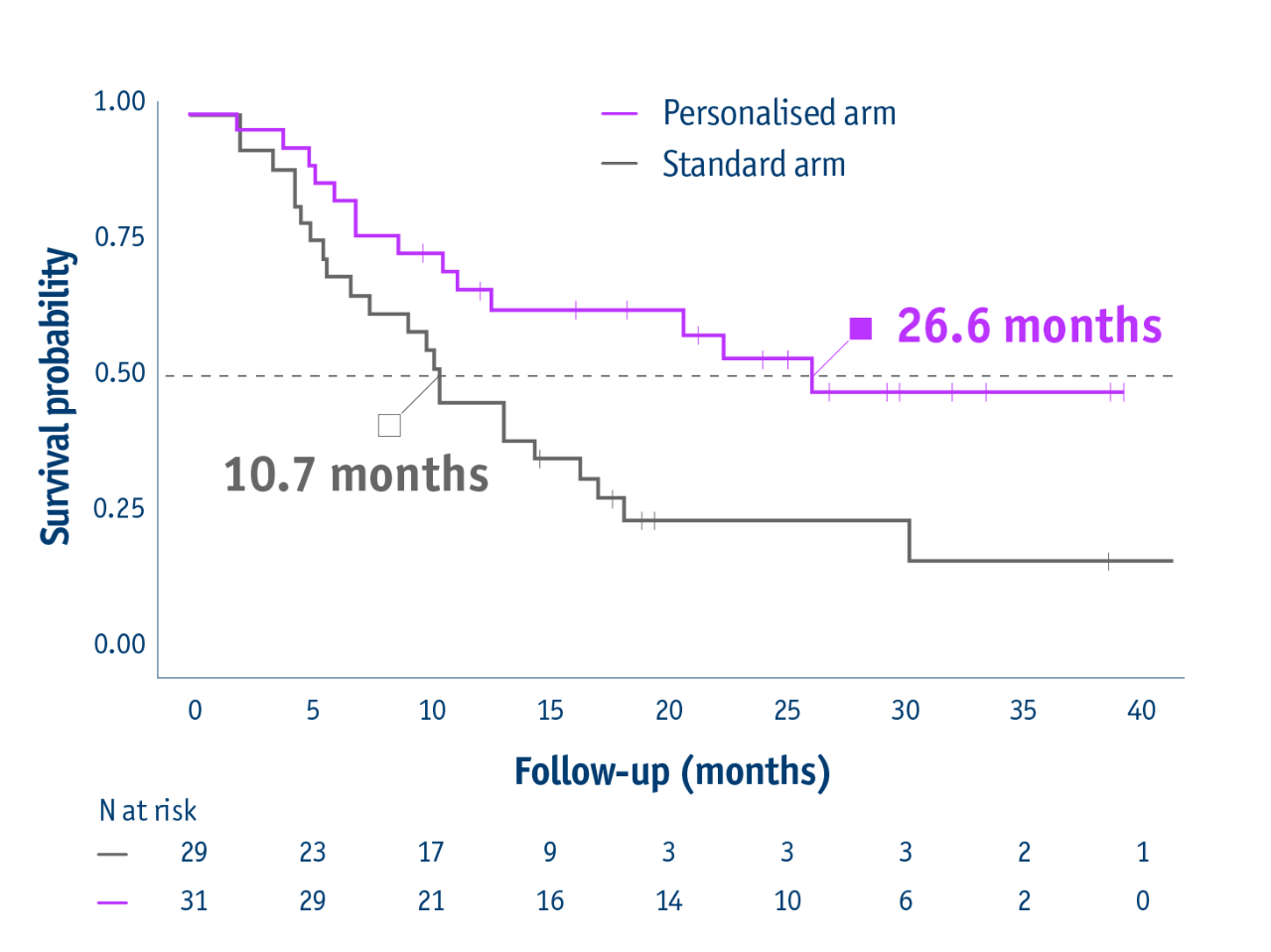
- 26.6 months median OS with personalised dosimetry
(95% CI: 11.7–not reached) - 10.7 months median OS in the standard dosimetry group
(95% CI: 6.0–16.8)
HR: 0.421 (95% CI: 0.215–0.826, P=0.0096)
23 months median OS for PVT patients in personalised arm vs 9.5 months in standard arm
16-month survival improvement
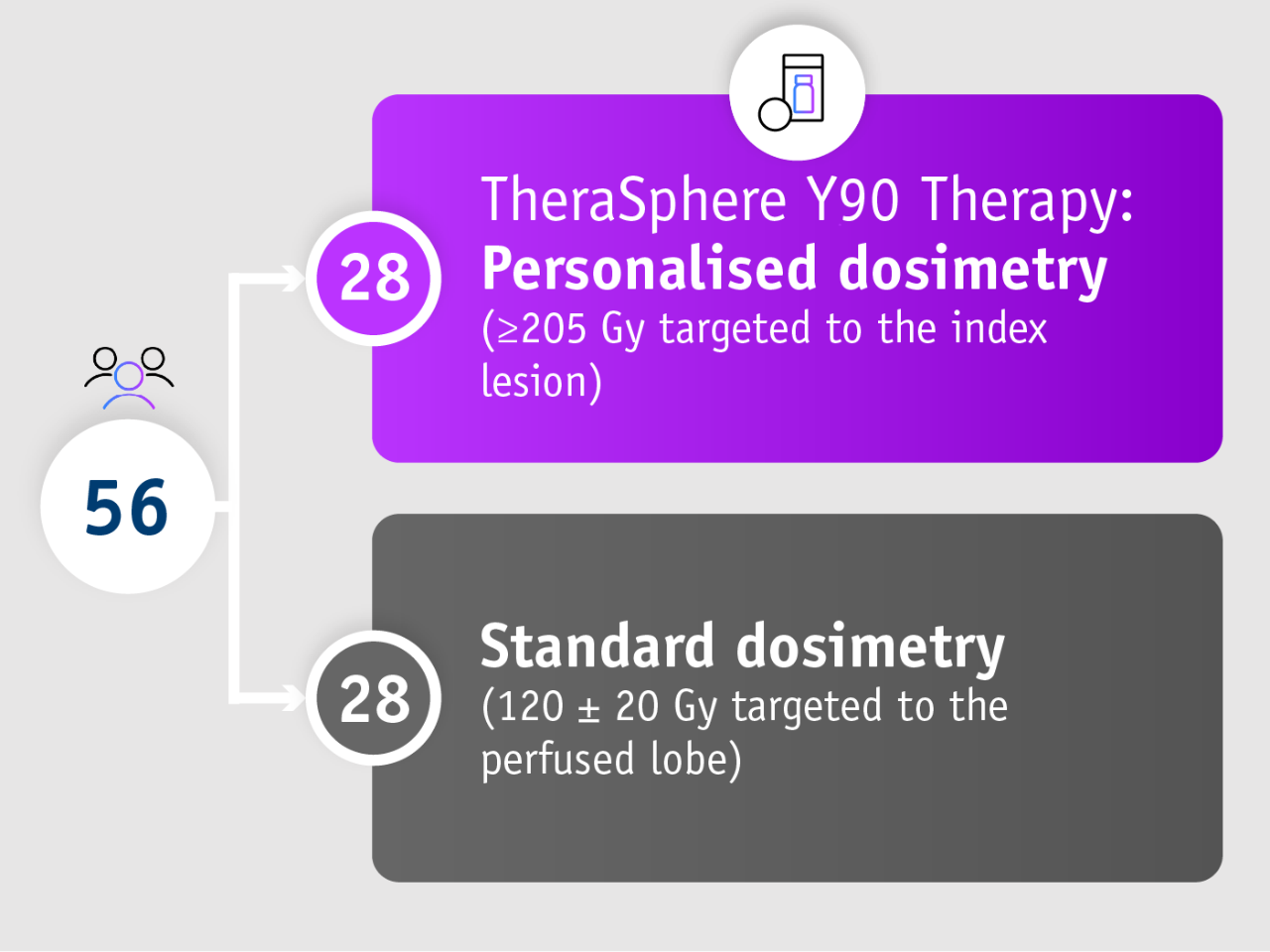
DOSISPHERE-01 trial (Garin et al. 2021)
• Phase II trial
• Randomised
• Multicentre (France)
• Open-label
Patients with unresectable locally advanced HCC
• ≥1 tumour ≥7 cm
• 90% BCLC C
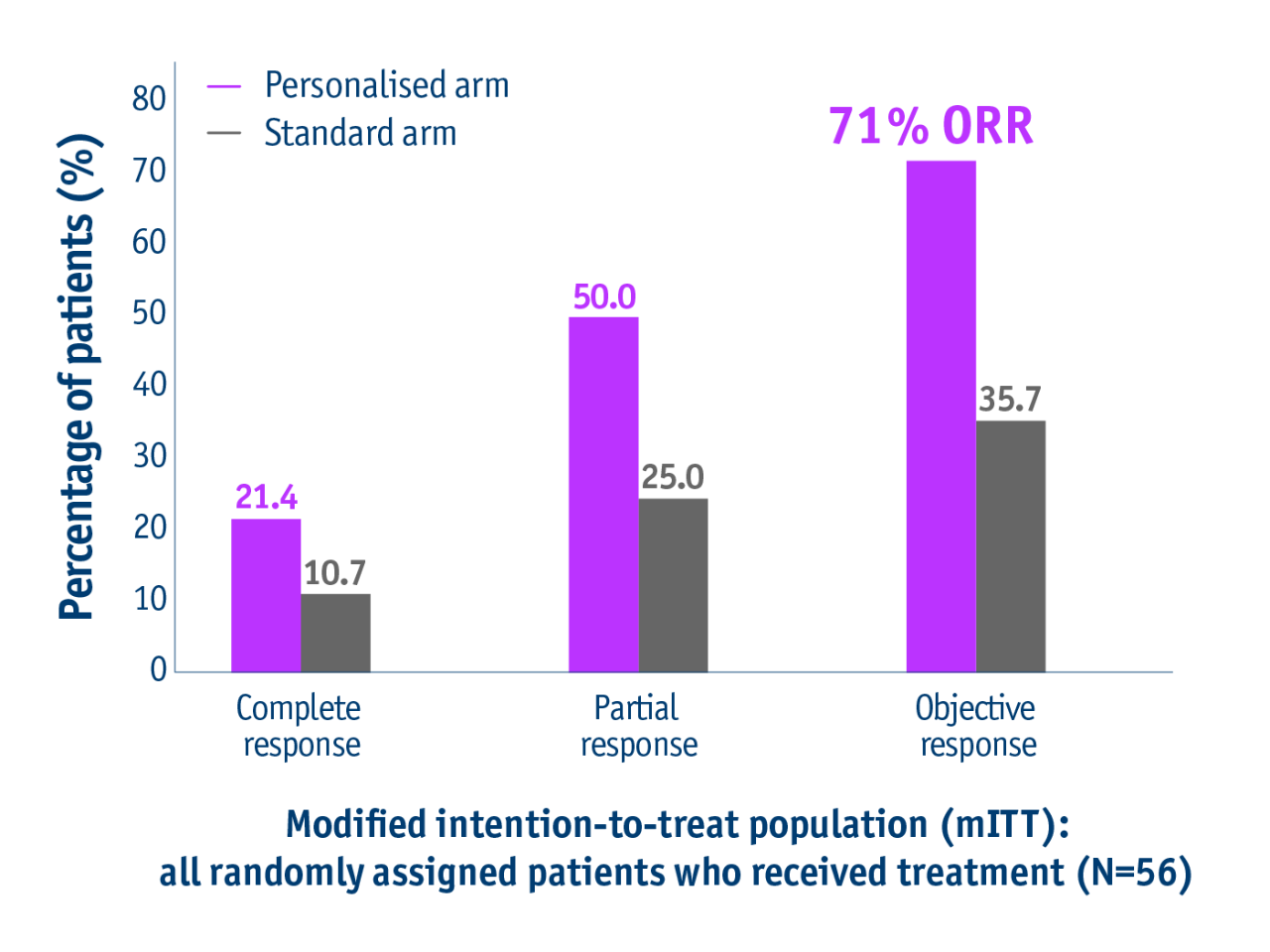
Offer better response rates
➣ Doubled response rates in patients with unresectable locally advanced HCC following personalised TheraSphereTM Y90 Therapy vs standard dosimetry2
Response rates in the DOSISPHERE-01 trial2
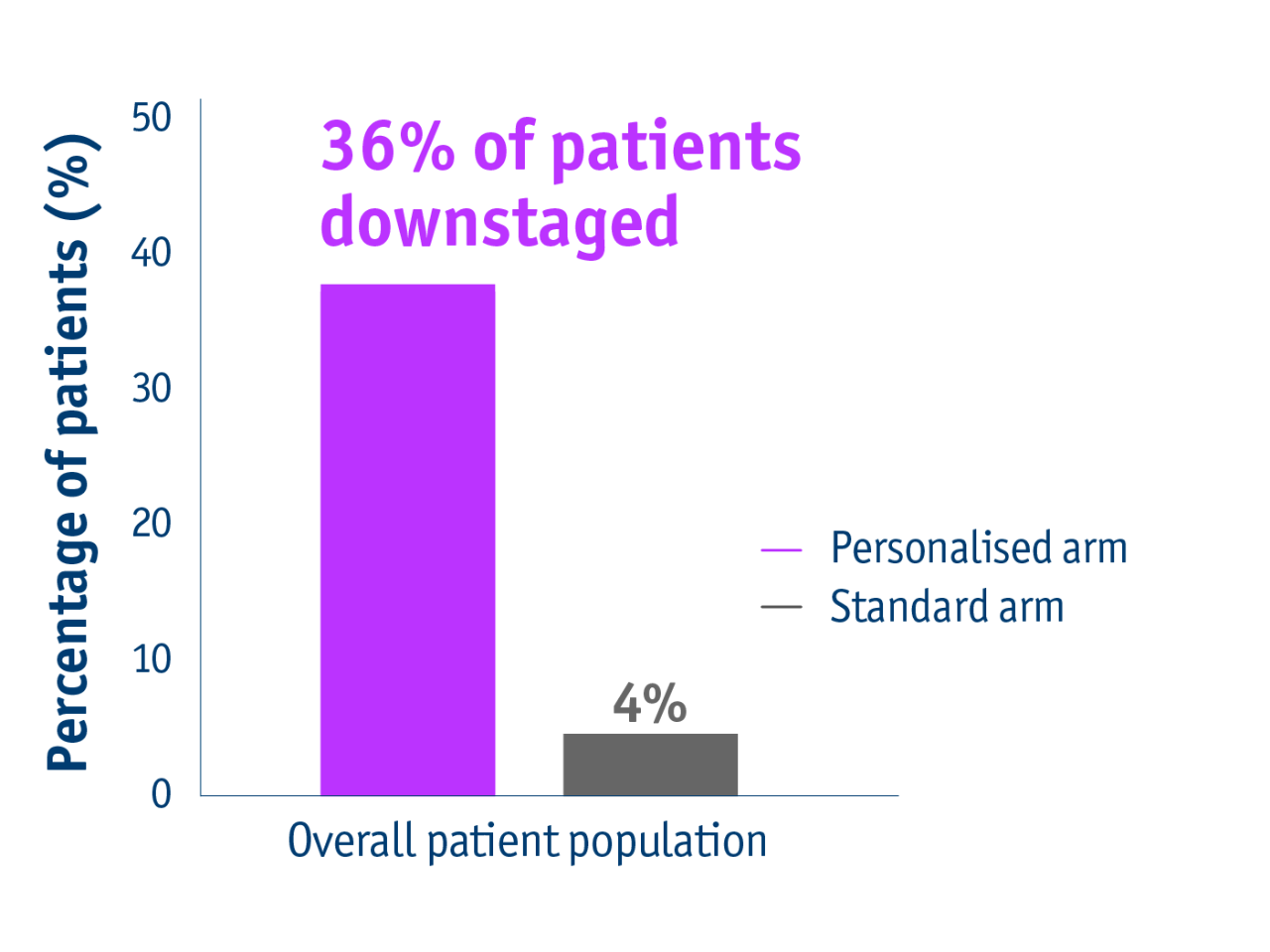
Promote curative options
➣ 36% of patients with unresectable locally advanced HCC accessed liver resection following personalised TheraSphereTM Y90 Therapy vs standard dosimetry2
Downstaging data in the DOSISPHERE-01 trial2
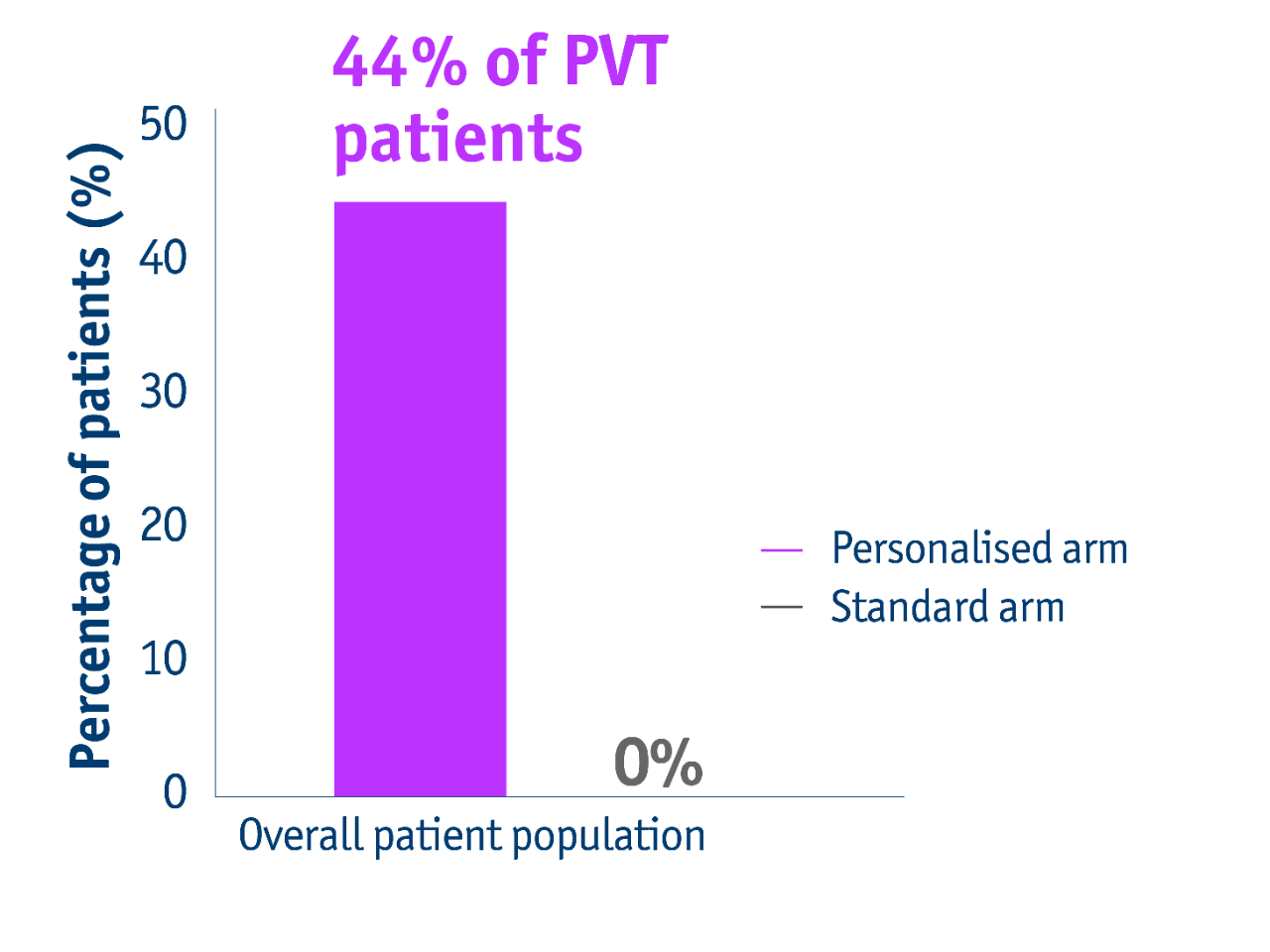
➣ 44% of PVT patients with unresectable locally advanced HCC accessed liver resection following personalised TheraSphereTM Y90 Therapy vs standard dosimetry2
Downstaging data for PVT patients in the DOSISPHERE-01 trial2
References
1.Pasciak AS, et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imaging. 2020 Apr;47(4):816-827.
2.Garin E, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021 Jan;6(1):17-29.
Abbreviations
AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; EASL, European Association for the Study of the Liver; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; ITT, intention to treat; mRECIST, modified Response Evaluation Criteria in Solid Tumors; ORR, objective response rate; OS, overall survival; PVT, portal vein thrombosis; RFS, recurrence-free survival; TACE, transarterial chemoembolisation; TTP, time to progression; UNOS, United Network for Organ Sharing; Y90, yttrium-90.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
















