SYNERGY™ & SYNERGY MEGATRON™
EES PtCr Coronary Stent System
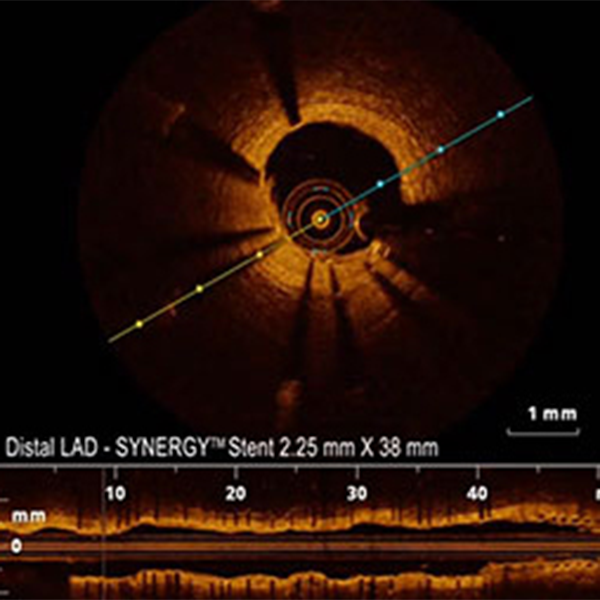
Boston Scientific now offers a portfolio of SYNERGY BP Stents designed to optimize performance and enable early healing in every type of case.

NOW CE Marked
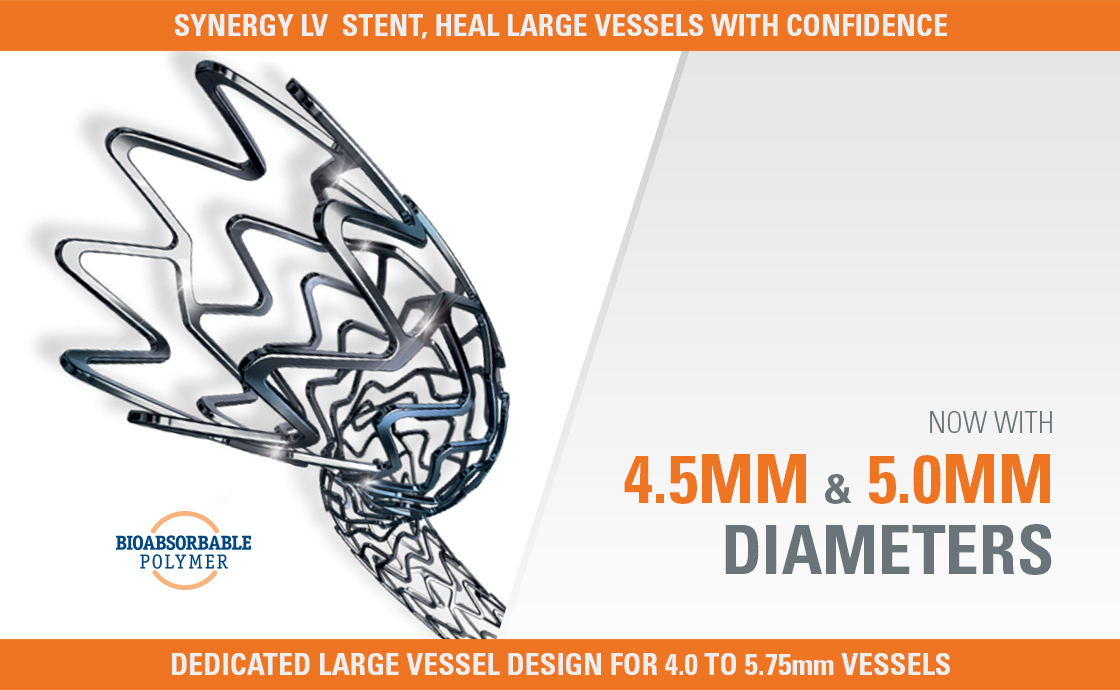
SYNERGY MEGATRON™
Purpose Built for Large Proximal Vessels