RANGER™
Paclitaxel-Coated PTA Balloon Catheter
Ranger SFA RCT
- Randomized, controlled trial
- Multicenter, 10 centers in Europe
- External, blinded core lab adjudication
- N = 105
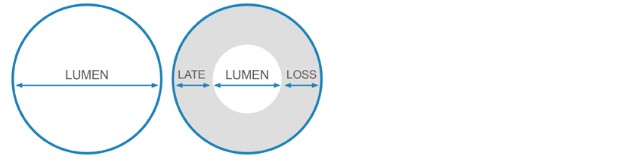
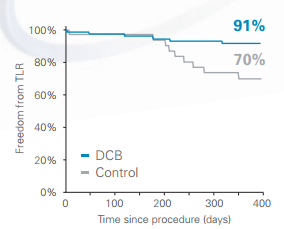
Freedom from TLR of 91% at 12 months, with significantly less late lumen loss vs. PTA.
Late Lumen Loss (mm)
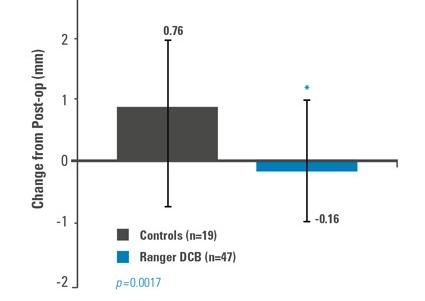
Late Lumen Loss
Freedom From TLR at 12 Months*
Similar Adverse Event and Serious Adverse Event rates between groups
- Ranger achieved a 91% Freedom from TLR at 12 months
- Ranger demonstrated Freedom from TLR superior* to PTA

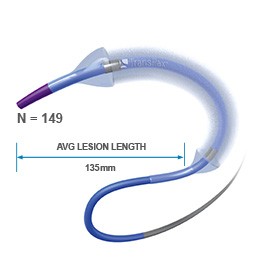
Ranger All Comers Registry¹
- Multicenter registry,
- Planned enrollment of 180 patients
- Average lesion length: 135 mm
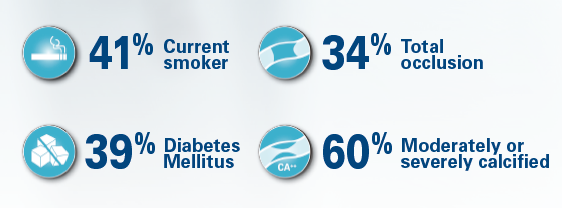
- Diabetes: 34 %
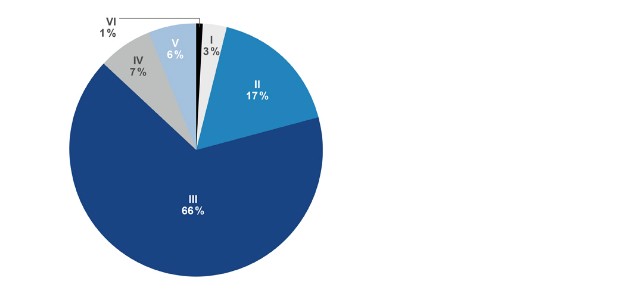
- Rutherford: 80 % Class III & higher
- TASC C&D: 59 %
- Percent diameter stenosis: 91 %
Kaplan-Meier Estimate
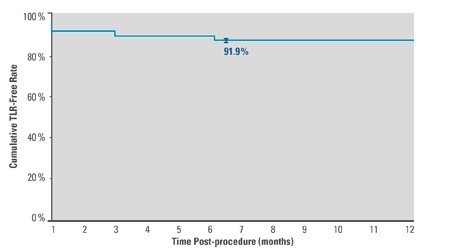
- 91.9%² freedom from TLR at 6 months
- 91.1%² primary patency at 6 months
- 91% of patients improved by at least 1 Rutherford category at 6 Months
- 80% of patients improved ≥2 Rutherford categories at 6 Months

Ranger Clinical Data Summary
- The Ranger DCB has been studied in over 250 patients, in both randomized and real-world clinical trials.
- The Ranger SFA-RCT has achieved among the highest FTLR of 91% and Primary Patency of 86% at 12 months.
- The Ranger All-Comers Registry confirms benefit of an efficient drug coating technology in challenging, real-world lesions with FTLR of 91.9%² and Primary Patency of 91.1%² at 6 months.
Ranger Drug-Coated Balloon JACC Results
New comparative Drug Coated Balloon study in JACC's July issue
Impact of Paclitaxel Dose on Tissue Pharmacokinetics and Vascular Healing: A Comparative Drug Coated Balloon Study in the Familial Hypercholesterolemic Swine Model of Superficial Femoral In-Stent Restenosis. C.A. Gongora MD and all.
Comparison of Different Drug Coated Balloons on Tissue Pharmacokinetics and Vascular Healing in In Stent Restenosis (ISR) Swine Model
Main conclusion:
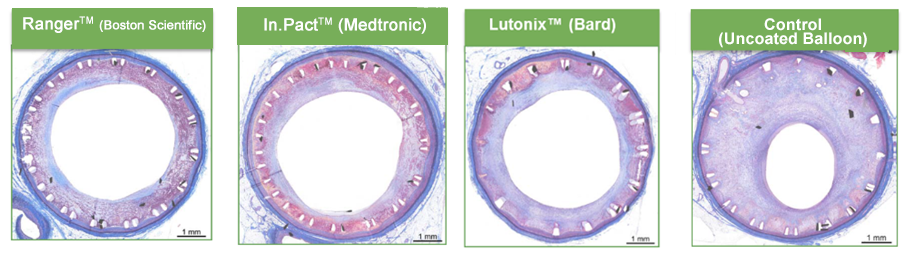
Compared to POBA (Plain old balloon angioplasty) all tested Drug-Coated Balloons demonstrated efficacy (decreased Neointimal inhibition thickness and percent stenosis). The In.Pact DCB provided slightly higher levels of neointimal inhibition but also reduced neointimal maturity and higher fibrin deposition.3
The Ranger DCB technology demonstrated sustained tissue retention at lower loading doses with less particulate formation, but with an efficacy profile comparable to the already clinically proven first-generation DCB technologies.