EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
The Evolution of S-ICD Technology
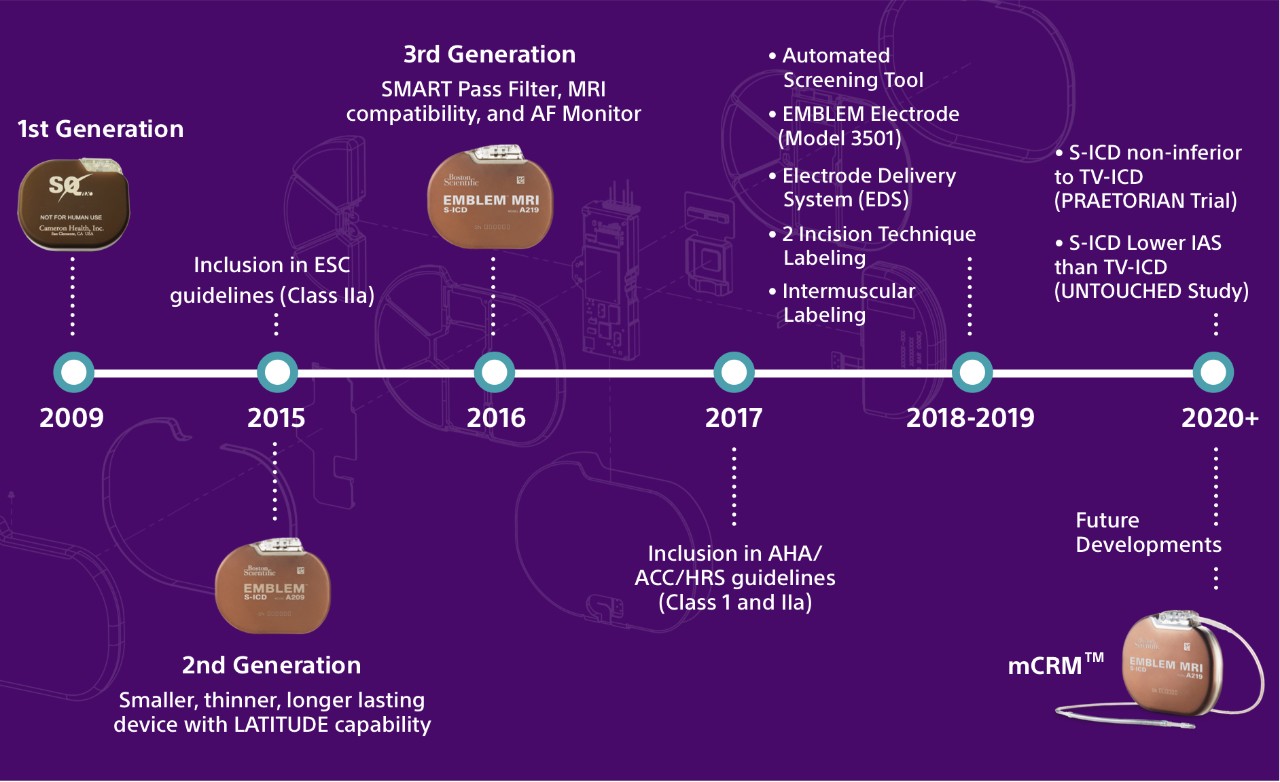
Next-Generation Technology: Key Features and Benefits
The EMBLEM MRI S-ICD is the second device in the EMBLEM S-ICD family and builds on previous enhancements in size, longevity and remote patient management. Like transvenous ICDs (TV-ICDs), the EMBLEM MRI S-ICD System uses a pulse generator capable of delivering life-saving therapy. Unlike TV-ICDs, the EMBLEM MRI S-ICD System leaves the heart and vasculature untouched, avoiding the risks and complications associated with transvenous leads.
Device Longevity
Real world data clinically shows EMBLEM S-ICD longevity of 8.7 years.1
SMART Pass
The SMART Pass filter is designed to reduce cardiac over-sensing and data has demonstrated that the inappropriate shock rate for S-ICD is now lower than TV-ICDs. Learn More About The UNTOUCHED Study.
AF Monitor
Designed to assist in the detection of silent, new onset or the progression of atrial fibrillation.2 AF Monitor will notify a clinician when at least six minutes of atrial fibrillation have been detected within a day.
LATITUDE™ NXT Remote Patient Management System
Remote patient management has been shown to decrease mortality, hospitalizations and in-clinic evaluations.3 The EMBLEM MRI S-ICD System leverages LATITUDE NXT remote monitoring technology to help improve patient outcomes and enhance clinic efficiencies.
EMBLEM MRI S-ICD Pulse Generator
| Mechanical Specifications | |
|---|---|
| Model Number | A219 |
| Size (W x H x D) | 83.1 x 69.1 x 12.7 mm |
Mass | 130 g |
Volume | 59.5 cc (cm³) |
Projected Longevity | 8.7 years* |
Battery Chemistry | Boston Scientific Li/MnO2 |
Warranty | 6 years** |
Remote Patient | Enabled for LATITUDE™ NXT Remote Patient Management |
| Reimbursement Information | S-ICD electrode C-Code:C1896 S-ICD pulse generator C-Code: C1722 |
**For full warranty terms and conditions go to www.bostonscientific.com/en-US/pprc/warranty-info-forms.html
| Automatic Functions | |
Sensing Configuration | Primary (ring to can), Secondary (tip to can), Alternate (tip to ring) |
Gain Selection | x1, x2 |
Rhythm Discrimination | INSIGHT™ algorithm automatically activated when the Conditional Shock Zone is programmed |
Shock Polarity | Standard (coil to can), reverse (can to coil) Automatically selects and stores last successful shock polarity |
Adaptive Shock Polarity | Shock polarity alters automatically after failed shock |
SMART Charge | Automatically extends initial detection time to allow self termination of non-sustained tachyarrhythmias |
| SMART Pass | High-pass filter designed to reduce oversensing while maintaining an appropriate sensing margin |
Internal Warning System | Audible tone alerts patient to elective replacement indicator, electrode impedance out of range, prolonged charge times, failed device integrity check. NOTE: Exposure to MRI scanning can cause a permanent loss of the Beeper volume. The physician and patient should weigh the benefit of the MR procedure against the risk of losing the Beeper. |
| Programmable Parameters | |
|---|---|
Shock Zone | 170 bpm - 250 bpm (steps of 10 bpm) |
Conditional Shock Zone | Off, On 170 bpm - 240 bpm (minimum 10 bpm less than Shock Zone) |
S-ICD System Therapy | Off, On |
Post-Shock Pacing | Off, On (50 ppm, max 30 sec, demand-based) |
Induction Capability | 1-10 sec (50 Hz/200 mA) |
Delivered Energy | 80J biphasic |
| AF Monitor | Off, On (model A219 only) |
| MRI Protection | Time-out (hours): 6, 9, 12, 24 |
| Diagnostics | |
|---|---|
Episode Storage | S-ECG storage for over 40 arrhythmic events (treated & untreated) |
| AF Episodes | 7 stored (model A219 only) |
Other Data | Electrode impedance |
EMBLEM™ S-ICD Electrode Model 3501
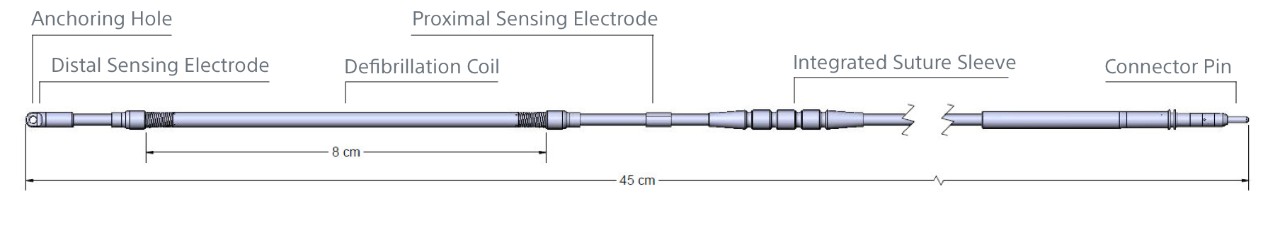
| Electrode Specifications | |
| Model Number | 3501 |
| Type | Tripolar |
| Length | 45 cm |
| Distal Type Size (Diameter) | 11.5 Fr / 3.84 mm |
| Coil Size (Diameter) | 9 Fr / 3 mm |
| Electrode Shaft Size (Diameter) | 7 Fr / 2.33 mm |
| Sensing Surface Area | Distal: 36 mm2 Proximal: 46 mm2 |
| Sensing Location | Distal: At tip Proximal: 120 mm from tip |
| Defibrillation Surface Area | 750 mm2 |
| Defibrillation Location | 20 - 100 mm from tip |
| Materials | Insulation: Polycarbonate, Polyurethane Electrodes: MP35N Conductors: MP35N Connector Pin: MP35N Integrated Suture Sleeve: Radiopaque White Silicone Slit Suture Sleeve: Silicone |
| Electrode C-Code | C1896 |
Training & Education

Explore continuing education courses, best practices modules and other training and resources for S-ICD.
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.
