EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
S-ICD is Guideline Recommended
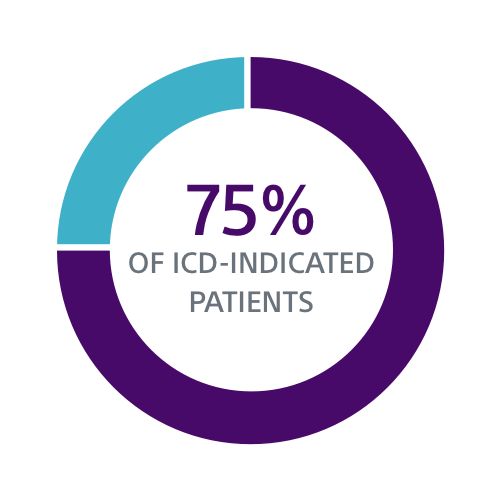
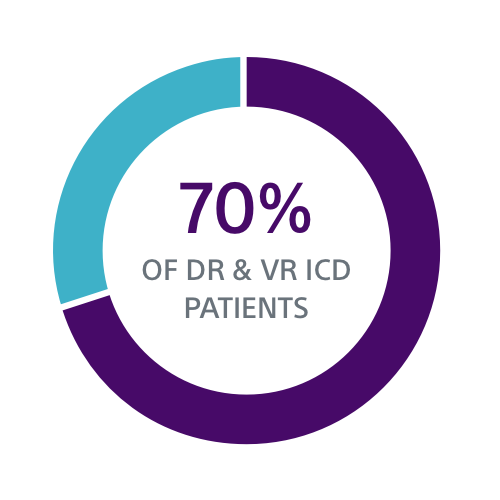
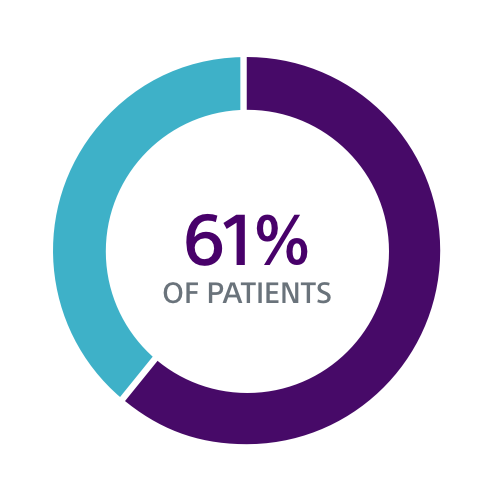
Clinical Data Supports S-ICD for a Broad Group of Patients
Both the PRAETORIAN and UNTOUCHED studies confirm S-ICD should be considered the first choice for all ICD-indicated patients without a pacing indication.
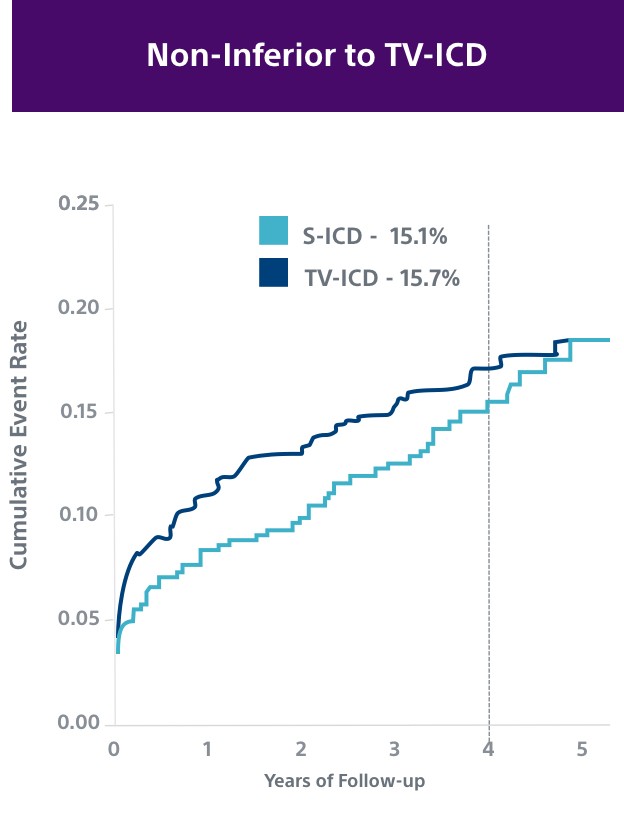
PRAETORIAN8
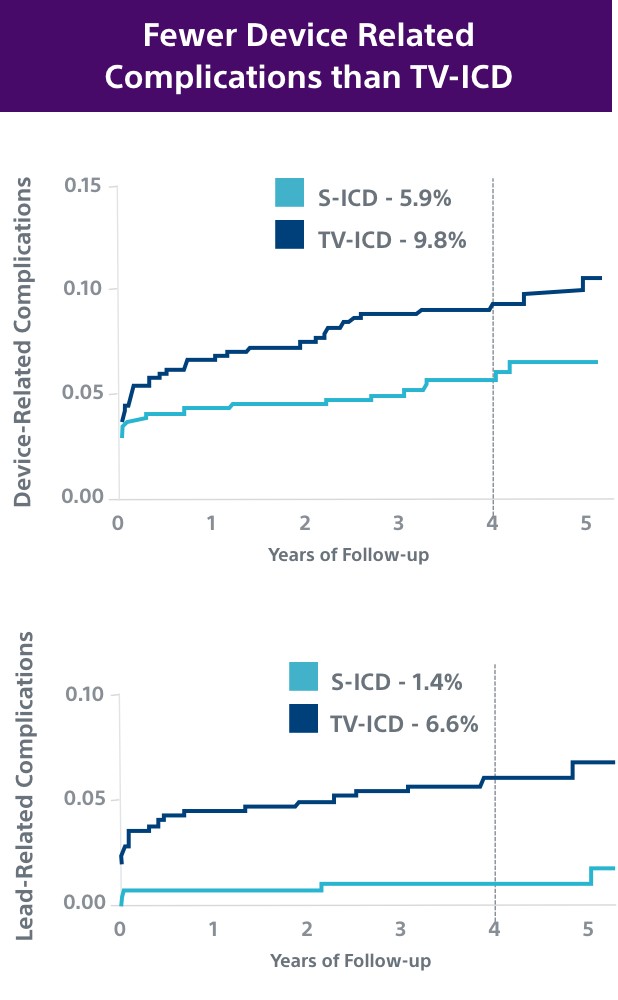
This investigator-sponsored trial demonstrated S-ICD had comparable performance to TV-ICD, despite including primarily older S-ICD devices and implant techniques. According to the PRAETORIAN study, S-ICD:
- Had significantly fewer lead-related complications (1.4% vs. 6.6%, P=0.001)
- Demonstrated fewer serious infections requiring extraction (8 TV-ICD vs. 4 S-ICD)
- Showed a trend in fewer overall complications (5.9% vs. 9.8%, P=0.11); likely to be significantly lower at 8 years in PRAETORIAN XL
UNTOUCHED9
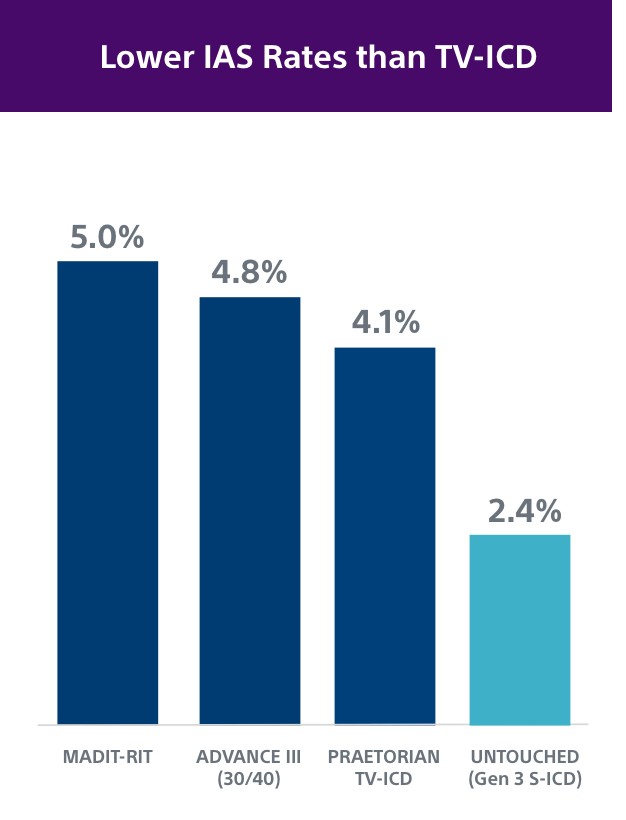
The UNTOUCHED study demonstrated patients with EMBLEM MRI S-ICD with SMART Pass had a 2.4% rate of inappropriate shocks at 1 year, which is as low or lower than TV-ICD devices.5,8,10-11
As S-ICD Technology Evolves, So Does the Clinical Data
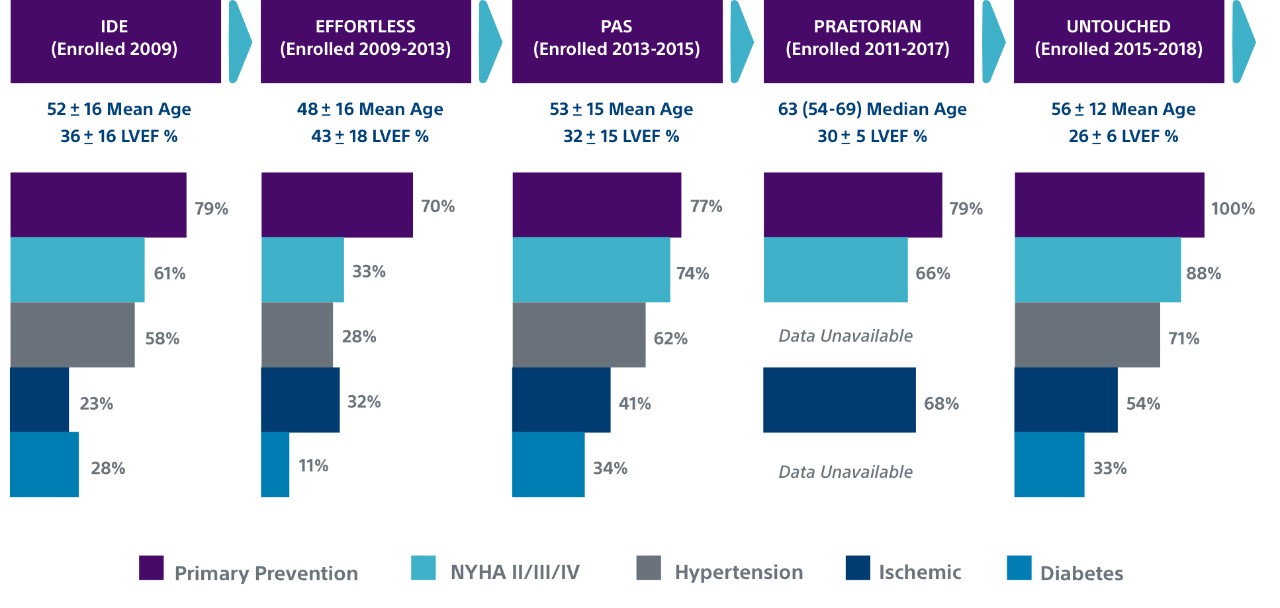
Over 82,000+ S-ICD patients and counting have received protection from both sudden cardiac death and the risks and complications associated with transvenous leads. Early S-ICD trials typically had younger patients with less advanced heart disease and “niche” indications, including channelopathies, hypertrophic cardiomyopathy, congenital heart disease or previous ICD indication. However, both the PRAETORIAN and UNTOUCHED studies included older and sicker patients. In fact, UNTOUCHED included the most traditional cohort of patients (primary prevention, LVEF ≤ 35%) and showed the S-ICD has lower inappropriate shock (IAS) rates than TV-ICDs.
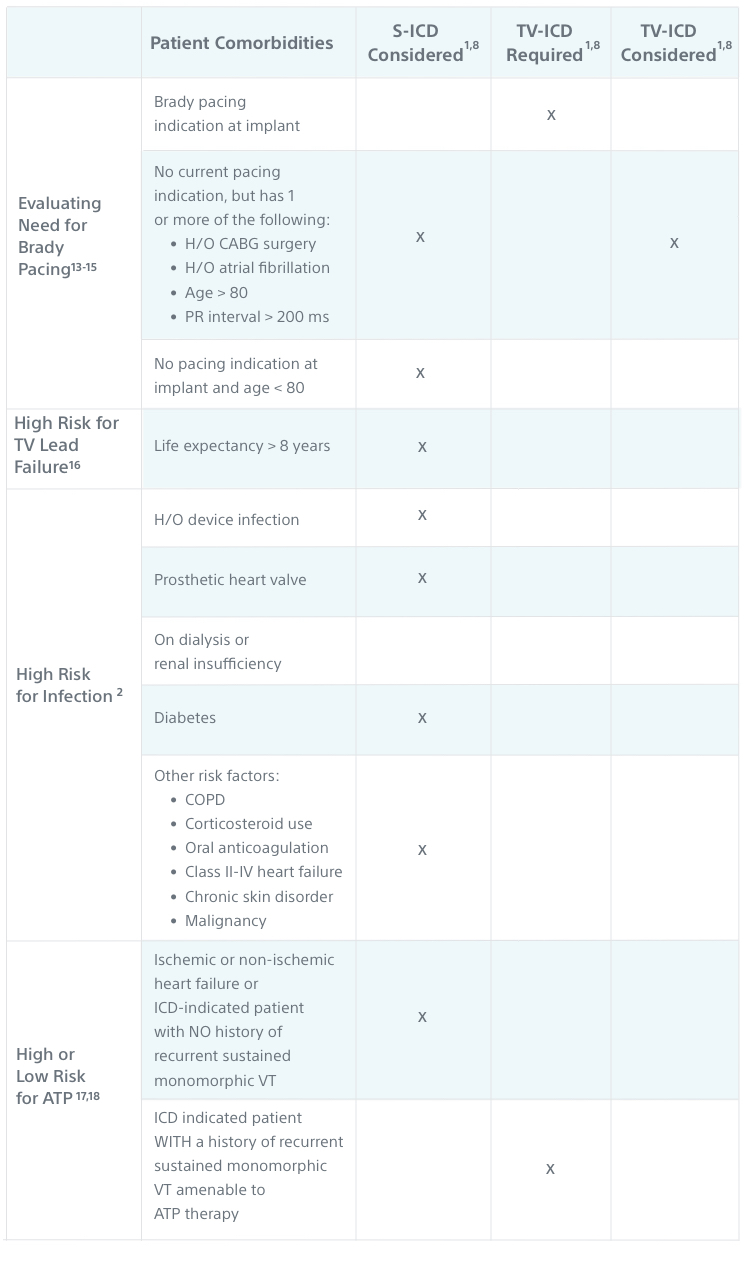
Patient Comorbidities and S-ICD
It’s important to consider patient comorbidities and other risk factors for complications when determining whether a TV-ICD is required or if a patient should be offered both S-ICD and TV-ICD when taking a shared decision-making approach to the treatment decision.
Patient Comorbidities |
S-ICD |
TV-ICD Required1,8 |
TV-ICD Considered1,8 |
|
|---|---|---|---|---|
| Evaluating Need for Brady Pacing13-15 | Brady pacing indication at implant |
|
X
|
|
No current pacing indication, but has 1 or more of the following:
|
|
X |
||
| No pacing indication at implant and age < 80 | X |
|
|
|
| High Risk for TV Lead Failure16 | Life expectancy > 8 years |
X |
|
|
| High Risk for Infection2 | H/O device infection | X |
|
|
| Prosthetic heart valve | X |
|
||
| On dialysis or renal insufficiency | X |
|
||
| Diabetes | X |
|
||
Other risk factors:
|
X |
|
||
| High or Low Risk for ATP17,18 | Ischemic or non-ischemic heart failure or ICD-indicated patient with NO history of recurrent sustained monomorphic VT |
X |
||
ICD-indicated patient WITH a history of recurrent sustained monomorphic VT amenable to ATP therapy |
X |
Physician Perspectives

Training & Education

Explore continuing education courses, best practices modules and other training and resources for S-ICD.
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.