RANGER II SFA Clinical Trial Results
Prospective, Multi-Center, Randomized Controlled Trial Ranger Drug-Coated Balloon vs. Uncoated Balloon (3:1). Follow-up through 5-Years.1
Ranger DCB demonstrated exceptional outcomes at 2-Years.
Ranger demonstrated the highest ever reported 2-Year Kaplan-Meier primary patency for a DCB in an RCT.
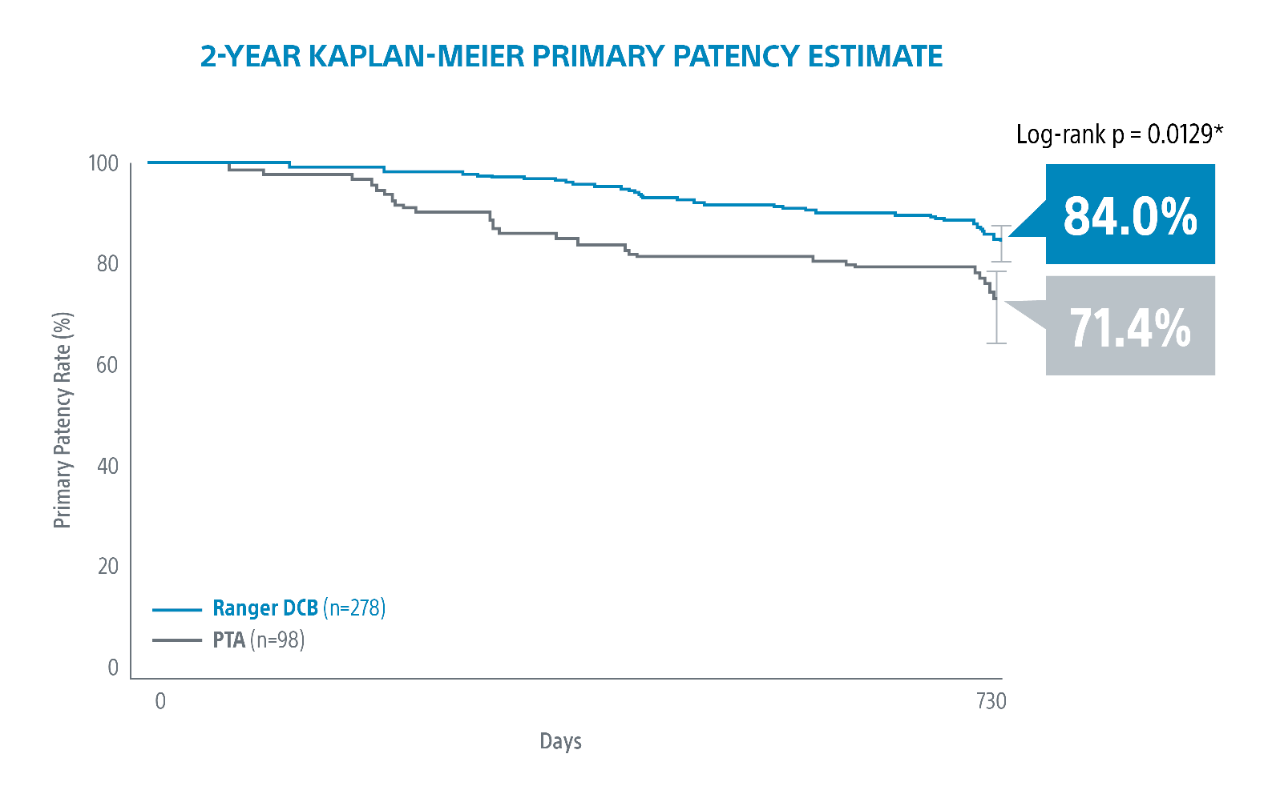
*Log-rank p-value compares the entire K-M curves from time point zero to day 760 (full 2-year follow-up window)
1. RANGER II SFA RCT 2-Year Results presented by Ravish Sachar, MD. VIVA 2021.2-year subgroup analysis
Ranger DCB delivered exceptional outcomes in complex lesions at 2-Years.
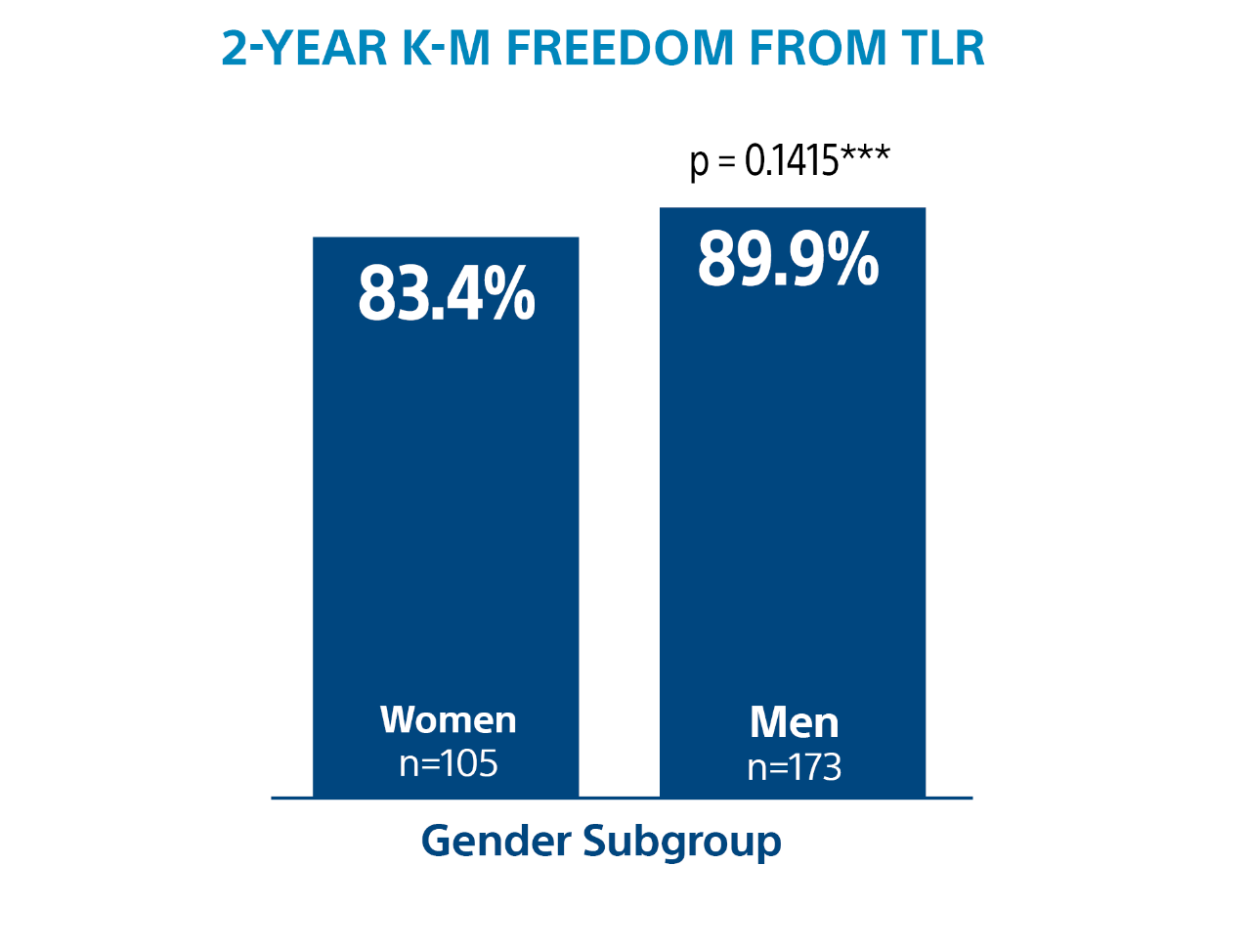
Ranger DCB demonstrated low reintervention rates regardless of patient gender.
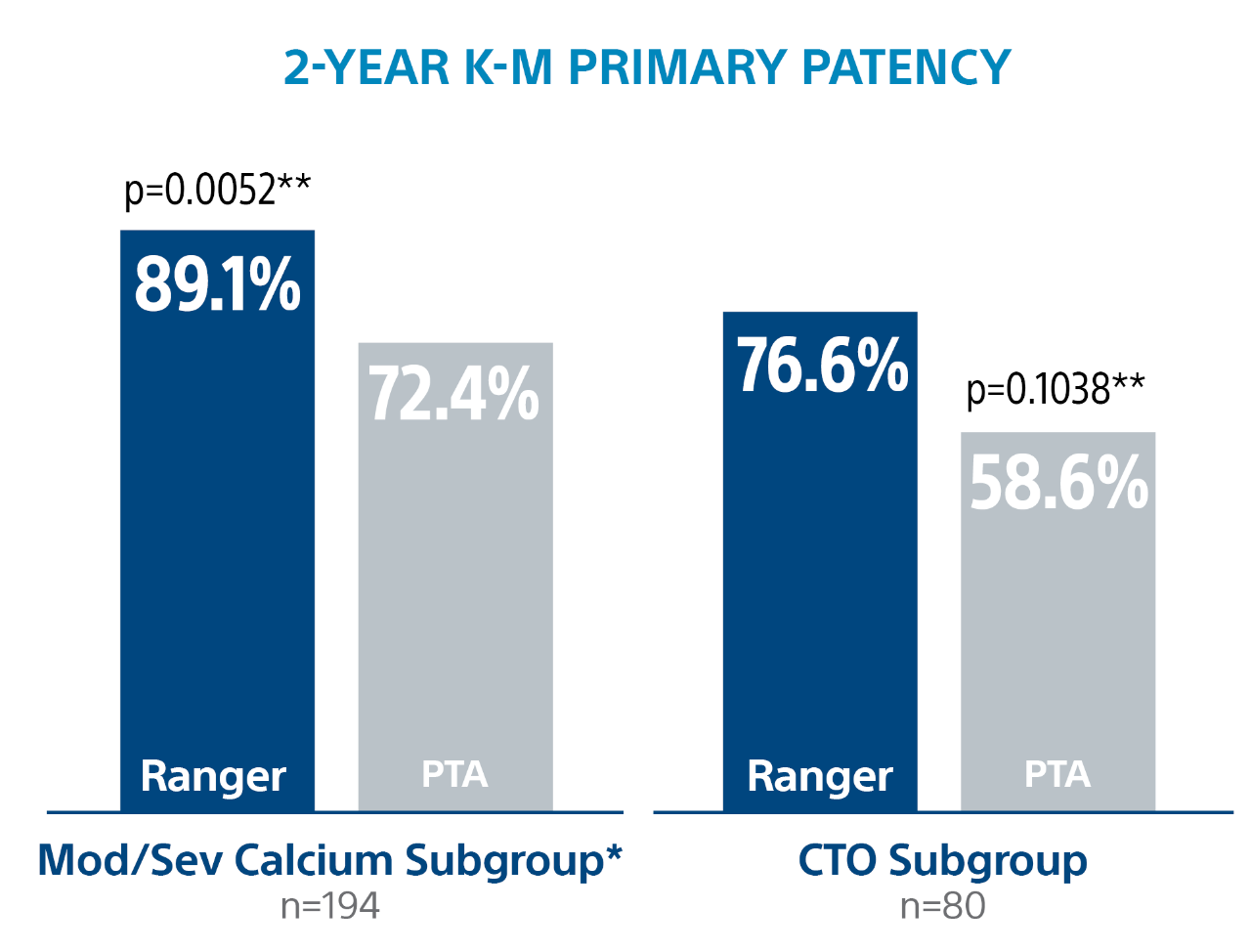
* PACSS Grade 3/4 Calcification
** Log-rank p-value compares the entire K-M curves from time point zero to day 760 (full 2-year follow-up window)
*** Log-rank p-value compares the entire K-M curves from time point zero to day 730 (full 2-year annual visit mark)
RANGER II SFA PK sub study²
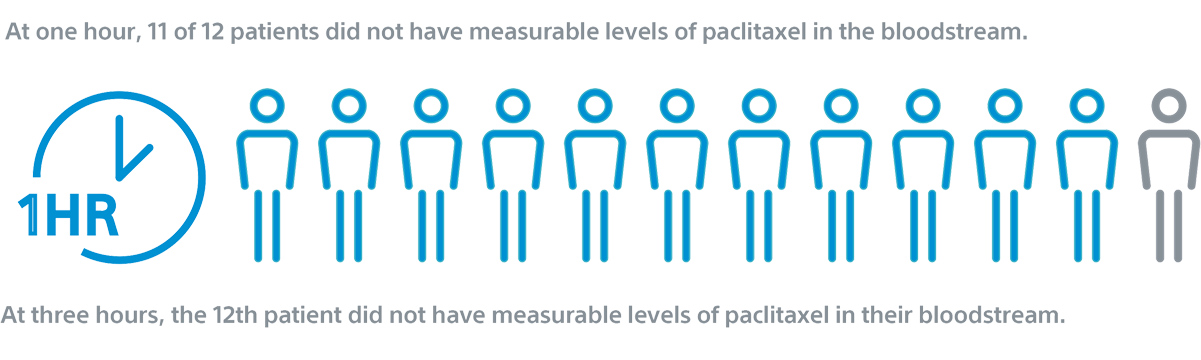
2. The limit of quantification was defined as < 1 ng/mL. RANGER II SFA PK Substudy. RANGER II SFA RCT 1-Year Results published in JACC:CI. doi.org/10.1016/j.jcin.2021.03.021.
Ranger II SFA pivotal trial details
- 1-year primary endpoint results
- Baseline patient & lesion characteristics
| Ranger DCB (n=207) | PTA (n=98) | p-value | |
|---|---|---|---|
| Primary safety endpoint (Freedom from MAE) | 94.1% (241/256) | 83.0% (76/91) | P non-inferiority ‹0.0001 |
| Primary effectiveness endpoint (Binary primary patency) | 82.9% (194/234) | 66.3% (57/86) | 0.0017 |
| Ranger DCB (n=278) | PTA (n=98) | p-value | |
|---|---|---|---|
| Age (year) | 70.6 | 69.1 | 0.1887 |
| Women | 37.8% | 31.6% | 0.2769 |
| Smoking history | 0.0303 | ||
| Current/previous | 31.3% / 54.0% | 45.9% / 38.8% | N/A |
| Never/unknown | 14.4% / 0.4% | 15.3% / 0.0% | N/A |
| Diabetes mellitus | 42.4% | 43.9% | 0.8055 |
| Lesion length (mm) | 82.5 | 79.9 | 0.655 |
| Moderate calcium (PACSS grade 3) | 36.3% | 52.0% | 0.006 |
| Severe valcium (PACSS grade 4) | 11.5% | 10.2% | 0.724 |
| 100% (occlusion) | 18.3% | 29.6% | 0.019 |
- 1-year key results
- 2-year key results
- 3 and 4-year key results
| Ranger DCB (n=278) | PTA (n=98) | p-value | |
|---|---|---|---|
| CD-TLR | 5.5% | 16.5% | 0.0011 |
| K-M all-cause mortality | 1.9% | 2.1% | 0.8794 |
| Ranger DCB (n=278) | PTA (n=98) | p-value | |
|---|---|---|---|
| K-M freedom from TLR | 87.4% | 79.5% | 0.0316* |
| Mod/Sev calcium subgroup K-M freedom from TLR | 90.9% | 79.6% | 0.0246* |
| CTO subgroup K-M freedom from TLR | 85.6% | 62.8% | 0.0172* |
| All-cause mortality | 5.7% | 3.2% | 0.4218 |
| Ranger DCB (n=278) | PTA (n=98) | p-value | |
| 3-year results | |||
| K-M primary patency | 77.40% | 73.50% | p=0.2555 |
| 4-year results | |||
| All-cause mortality | 14% (39/278) | 12.2% (12/98) | p=0.6574 |
| K-M freedom from CD-TLR | 78.70% | 74.50% | p=0.2108 |
| Major Amputation | 0.00% | 0.00% | p-undefined |
* Log-rank p-value compares the entire K-M curves from time point zero to day 730 (full 2-year annual visit mark)
Primary safety endpoint: composite of freedom from device and procedure-related death through 30 days and freedom from major target limb amputation and CD-TLR through 1-Year post index-procedure.
Primary efficacy endpoint: primary patency at 1-Year defined as absence of clinically driven target lesion revascularization (CD-TLR) or binary restenosis determined as a peak systolic velocity ratio > 2.4 evaluated by duplex ultrasound core laboratory analysis.
CD-TLR: a reintervention performed for ≥ 50% diameter stenosis (confirmed by angiography) within ± 5 mm proximal and/or distal to the target lesion after documentation of recurrent clinical symptoms of PAD (increase of 1 Rutherford class or more) and/or drop of ABI (≥20% or >0.15 when compared to maximum early post-procedural level).
















