Offer a life-extending and tolerable treatment for patients with recurrent liver cancer1,3

Many HCC patients are faced with minimal options at diagnosis. TheraSphere Y90 Therapy can help your patients gain access to curative treatment options, no matter what the BCLC stage.
Offer significant survival benefits
Patients who are ineligible for conventional curative therapy
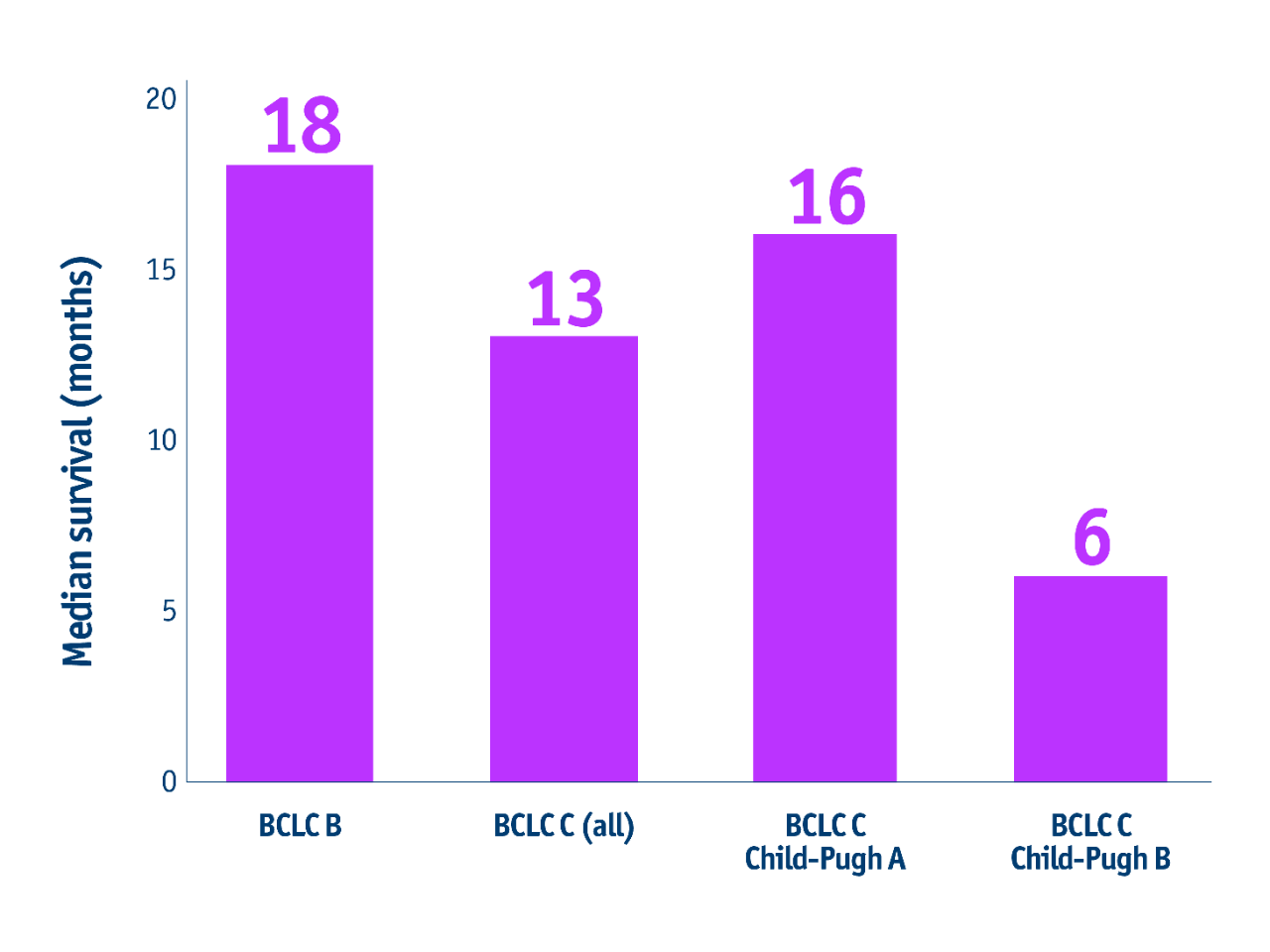
➣ 15 months median OS for patients with intermediate HCC (no PVT) or advanced HCC (with PVT) following TheraSphere Y90 Therapy3
Survival benefits in Mazzaferro et al. 20133
- Overall survival
- Summary
- Study design
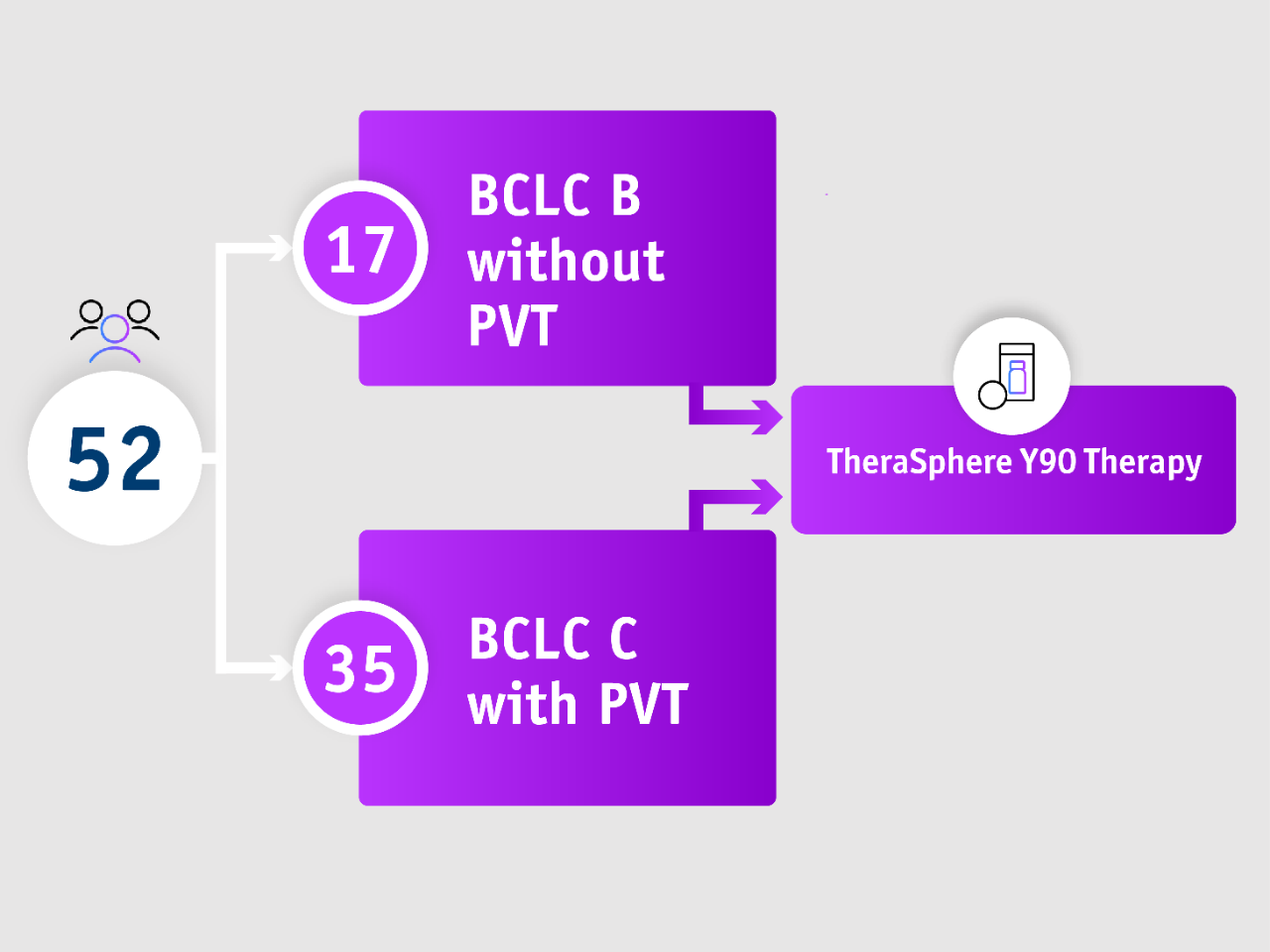
67% of patients had advanced HCC, all of which had PVT
• No gastrointestinal ulcers or pulmonary toxicities reported
• Zero treatment-related death and grade 3-4 bilirubin toxicities remained below 14% at 3 months
Mazzaferro et al. 2013
• Prospective, single centre, Phase II trial
• Patients with intermediate HCC (no PVT) or advanced HCC (with PVT) and well-compensated cirrhosis, (N=52)
Extend mOS* to 12.5 years post liver transplant*
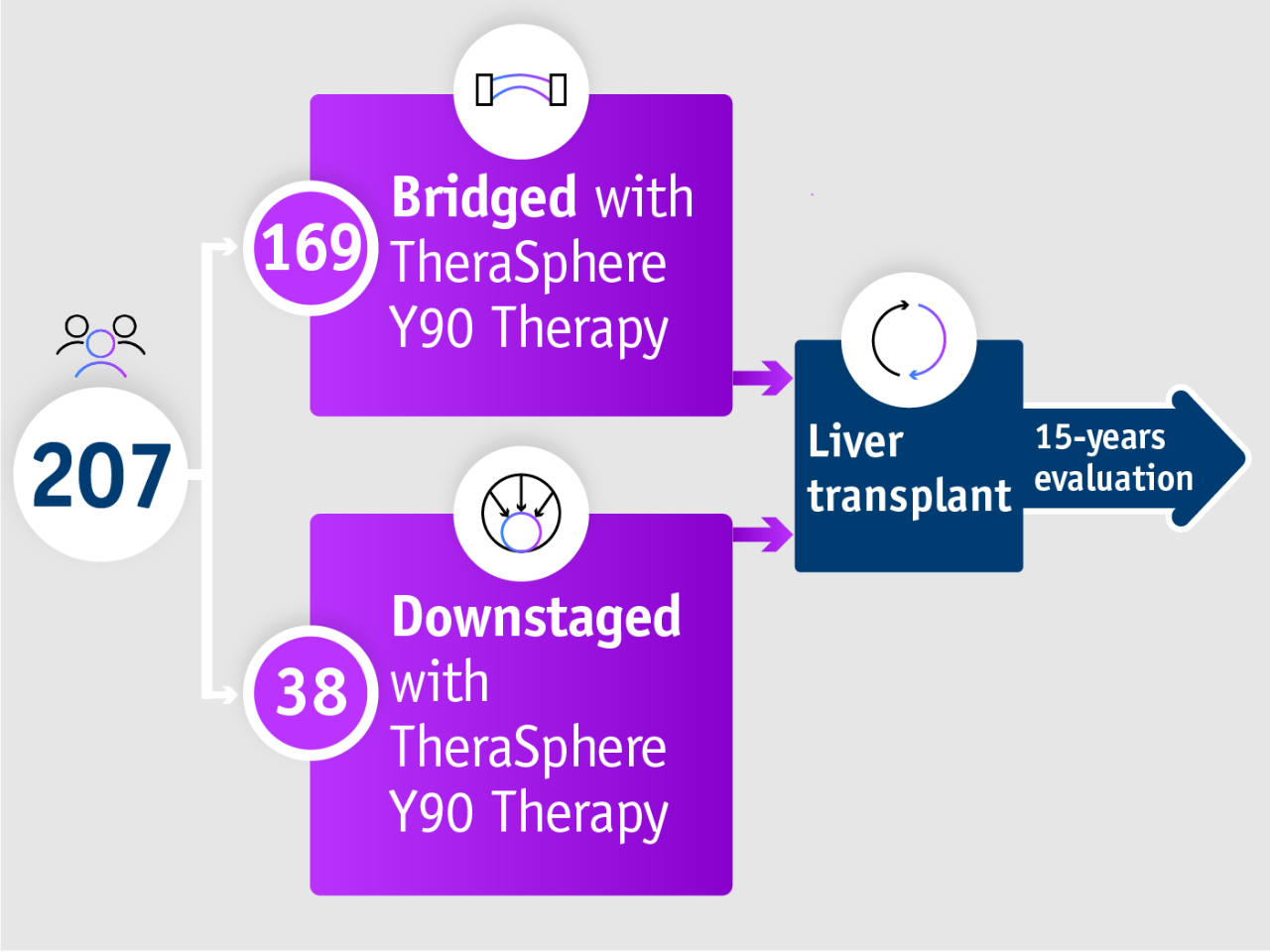
Patients who undergo liver transplant following bridging or downstaging treatment
➣ 12.5 years median OS post-LT following bridging or downstaging with TheraSphere Y90 Therapy1
15-year data from Gabr et al. 20211
- Overall survival
- Summary
- Study design
Overall survival of transplant patients
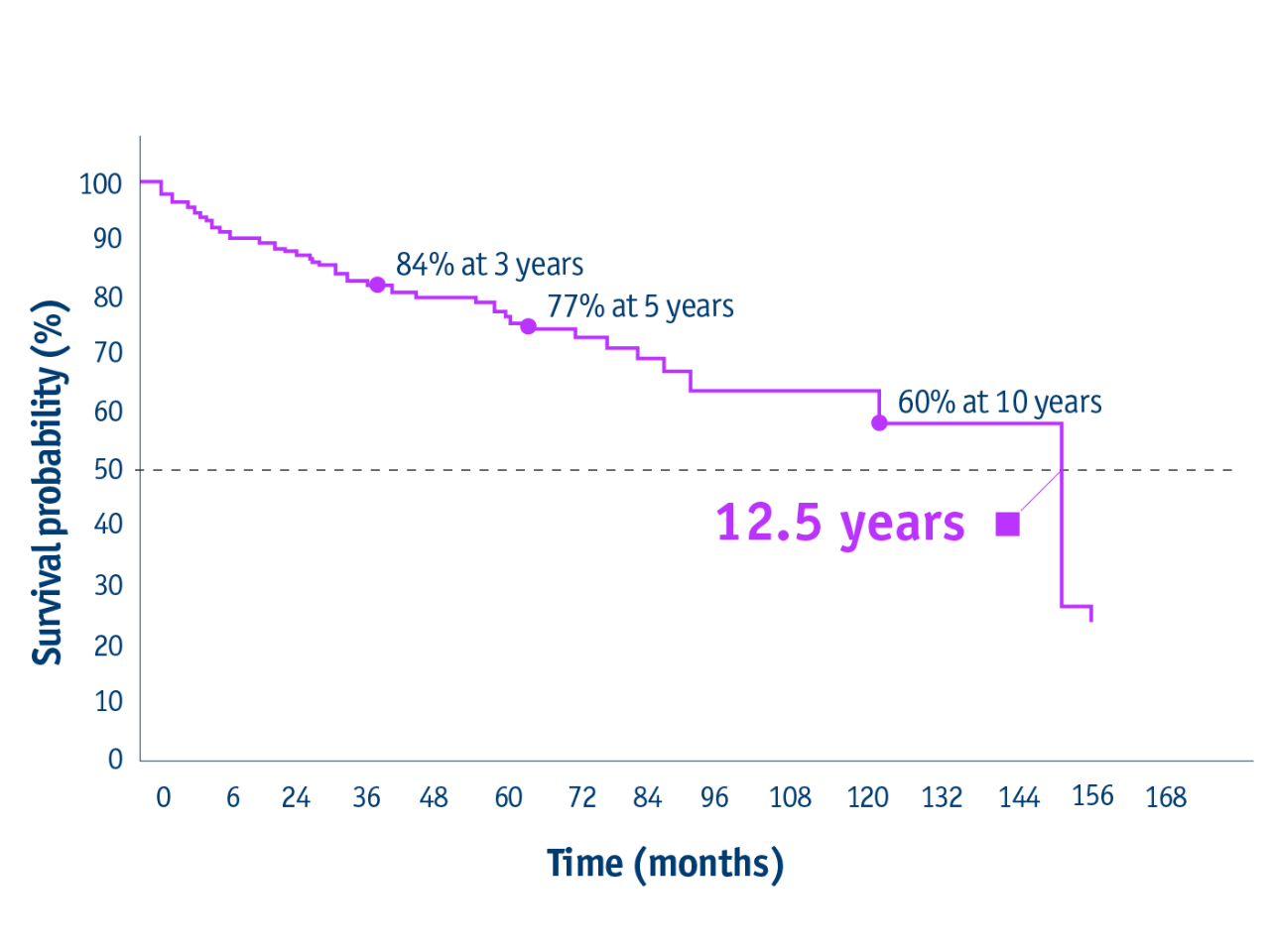
- 12.5 years median OS following liver transplant
(95% CI: 120–150)
Gabr et al. 2021
• Phase II trial
• Randomised
• Prospective
• Open-label
• Single-centre (USA)
Patients with HCC undergoing liver transplant following downstaging or bridging with TheraSphere Y90 Therapy
• Treatment-naïve (79.5%)
• 51% BCLC A; 31% BCLC C
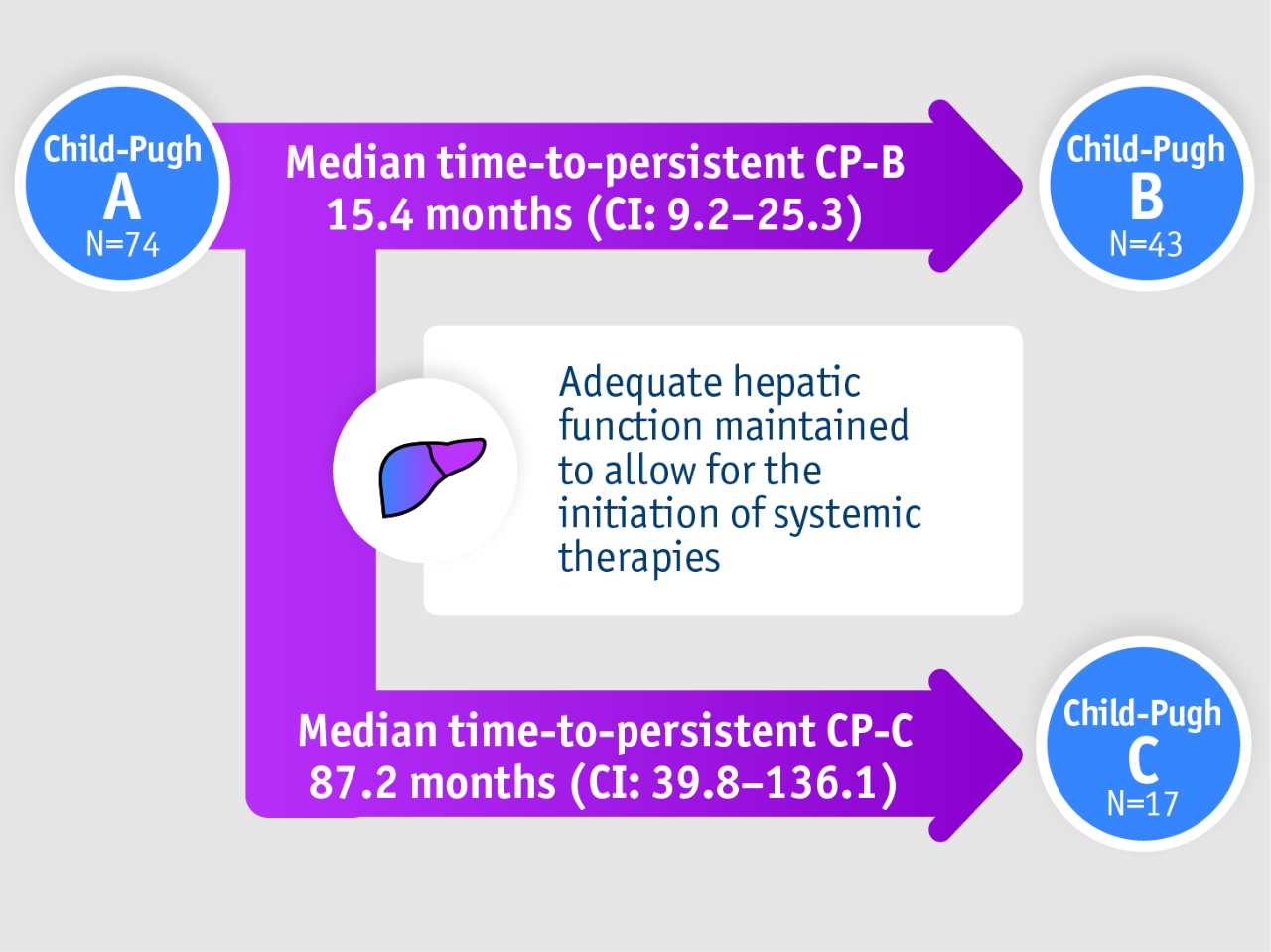
Patients with intermediate stage HCC Child-Pugh A
➣ 15.4 months of adequate liver function with TheraSphere Y90 Therapy, supporting continued treatment with systemic therapy4
Retrospective review, Ranganathan et al. 20224
- Time to persistent CP-B and C
- Overall survival
- Summary
- Study design
Overall survival (censored for transplant)
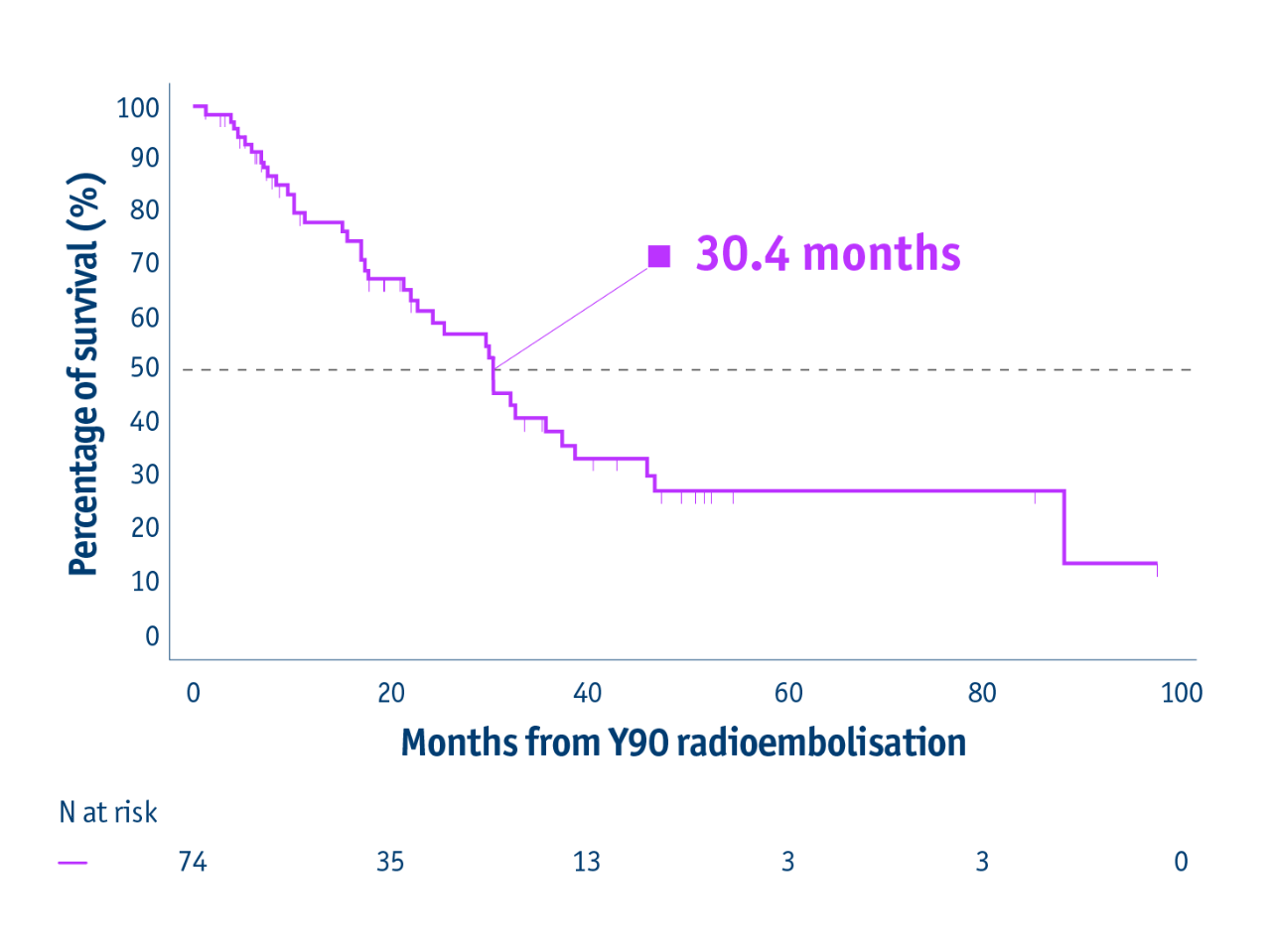
- 30.4 months mOS in BCLC B CP-A HCC patients
(CI: 22.7–37.4)
Ranganathan et al. 2022
Retrospective review of a prospectively acquired HCC/TARE database (2004–2017)
• N=74
• BCLC B/Child-Pugh (CP)-A
• (62%) bilobar disease
Extend life for HCC patients
Patients at all BCLC stages
➣ Extend mOS with TheraSphere Y90 Therapy as primary treatment5
Large single-centre HCC cohort: Salem et al. 20185
- Life-extending
- Summary
- Study design
Median overall survival (months)
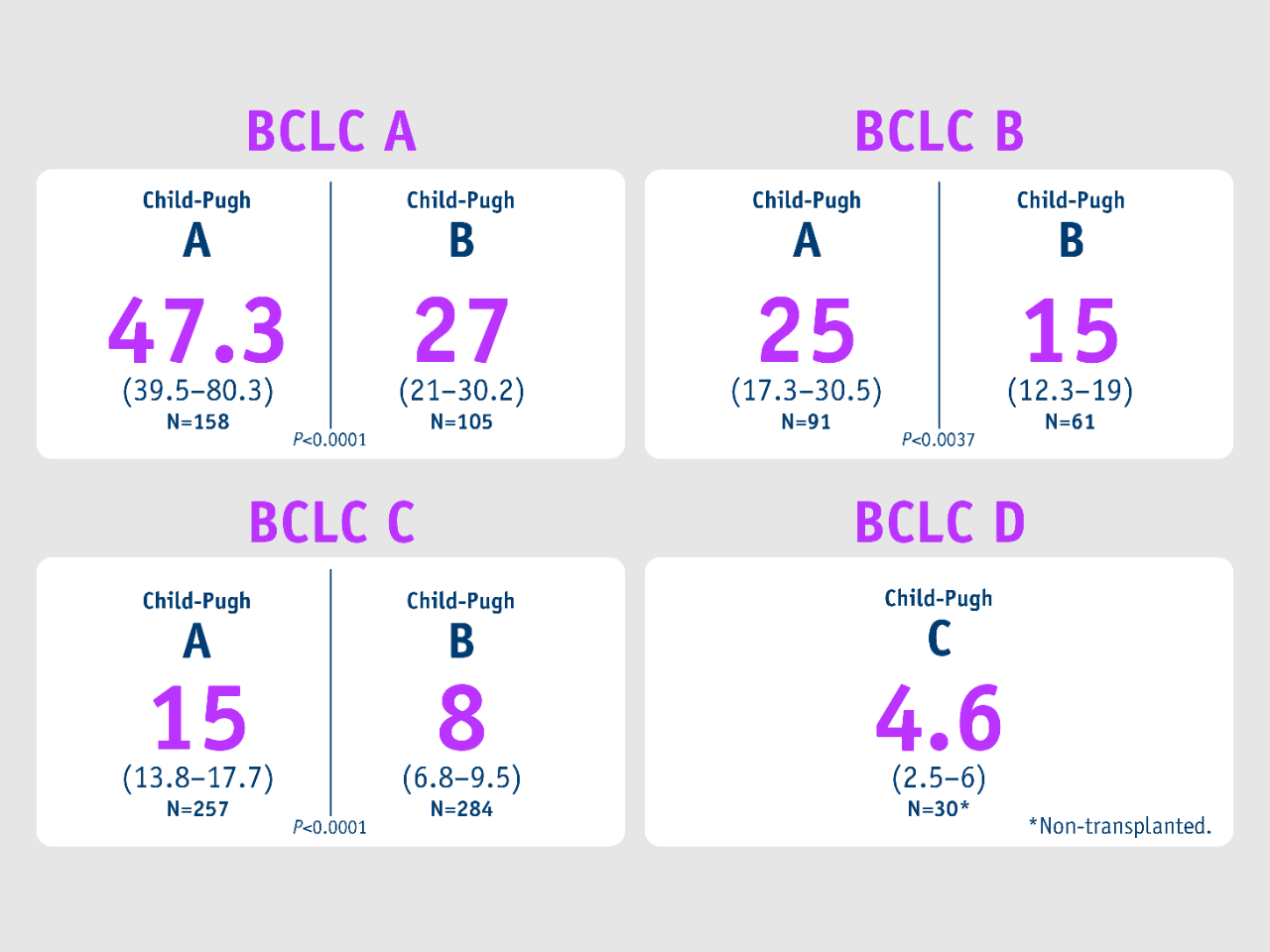
Salem et al. 2018
• From 2003 to 2017
• 1,000 HCC patients were treated with Y-90 glass microspheres as part of a prospective cohort study (the largest single-centre cohort conducted) with 1,577 total treatments (median: 1, range: 1-8)
Baseline characteristics:
• ECOG: 0 (56%); 1 (40%); 2 (4%)
• Child-Pugh: A (51%); B (45%); C (4%)
• BCLC: A (26%); B (15%); C (54%); D (4%)
Gain access to curative surgery
Patients that have only been offered palliative care
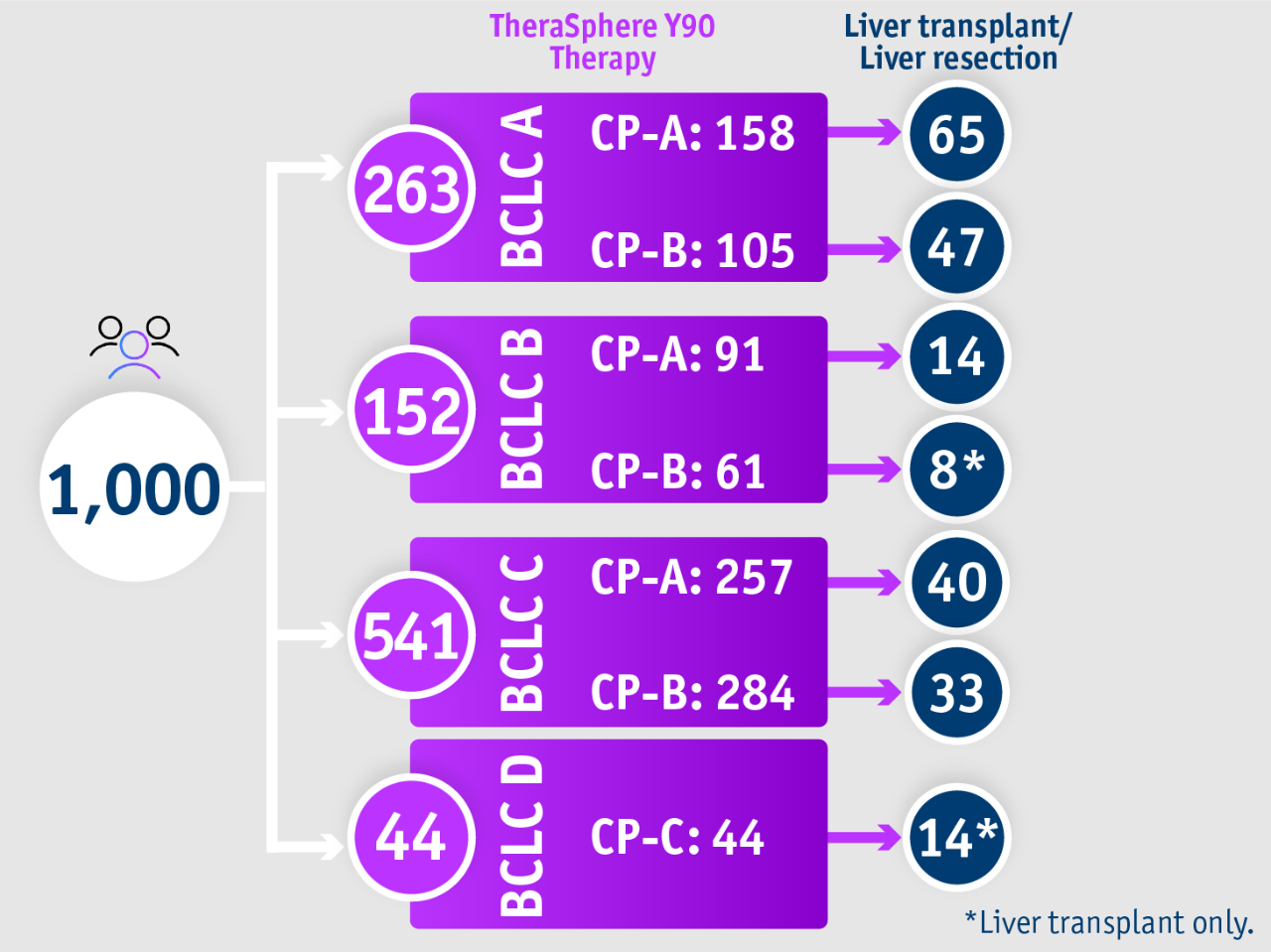
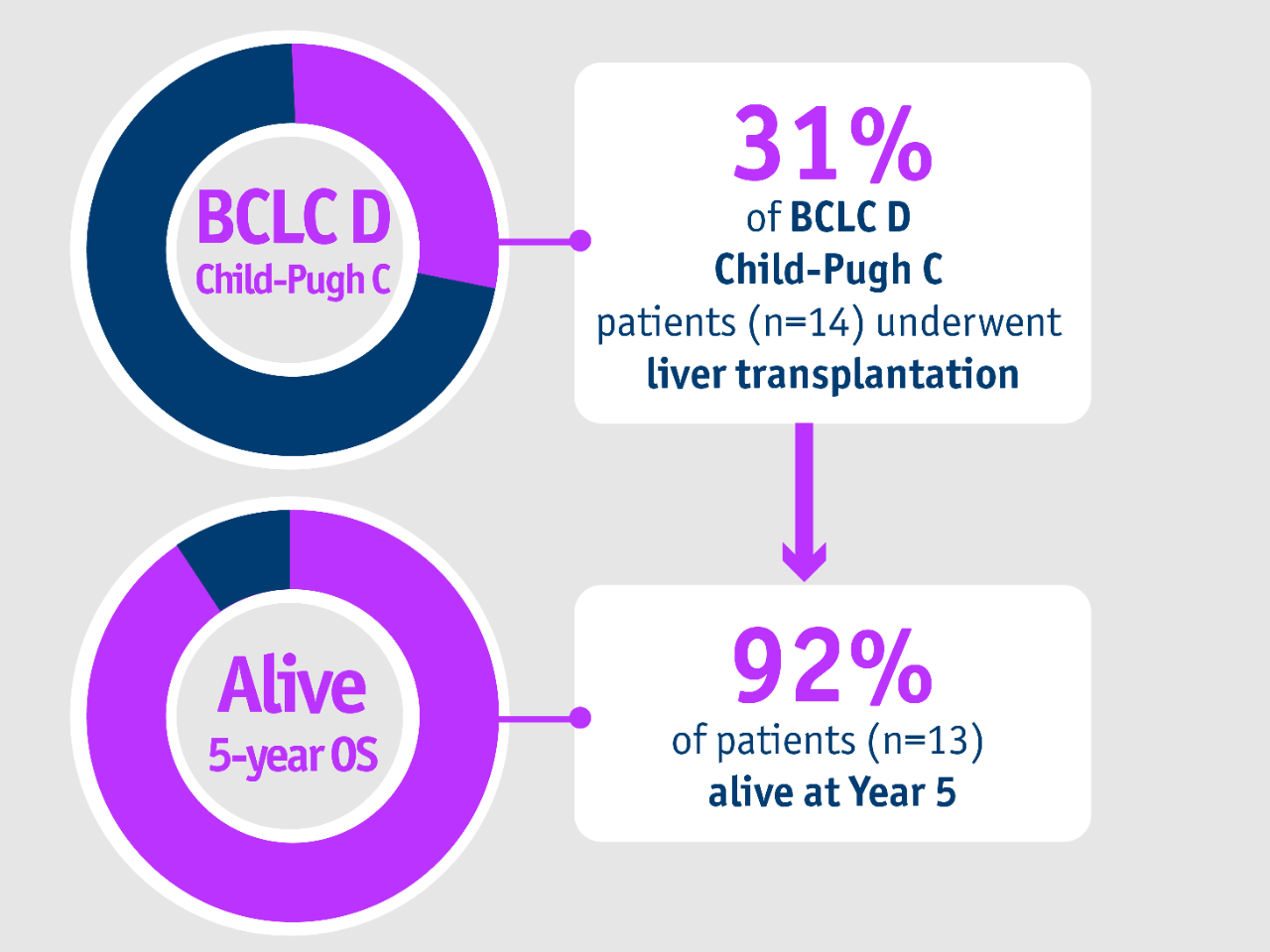
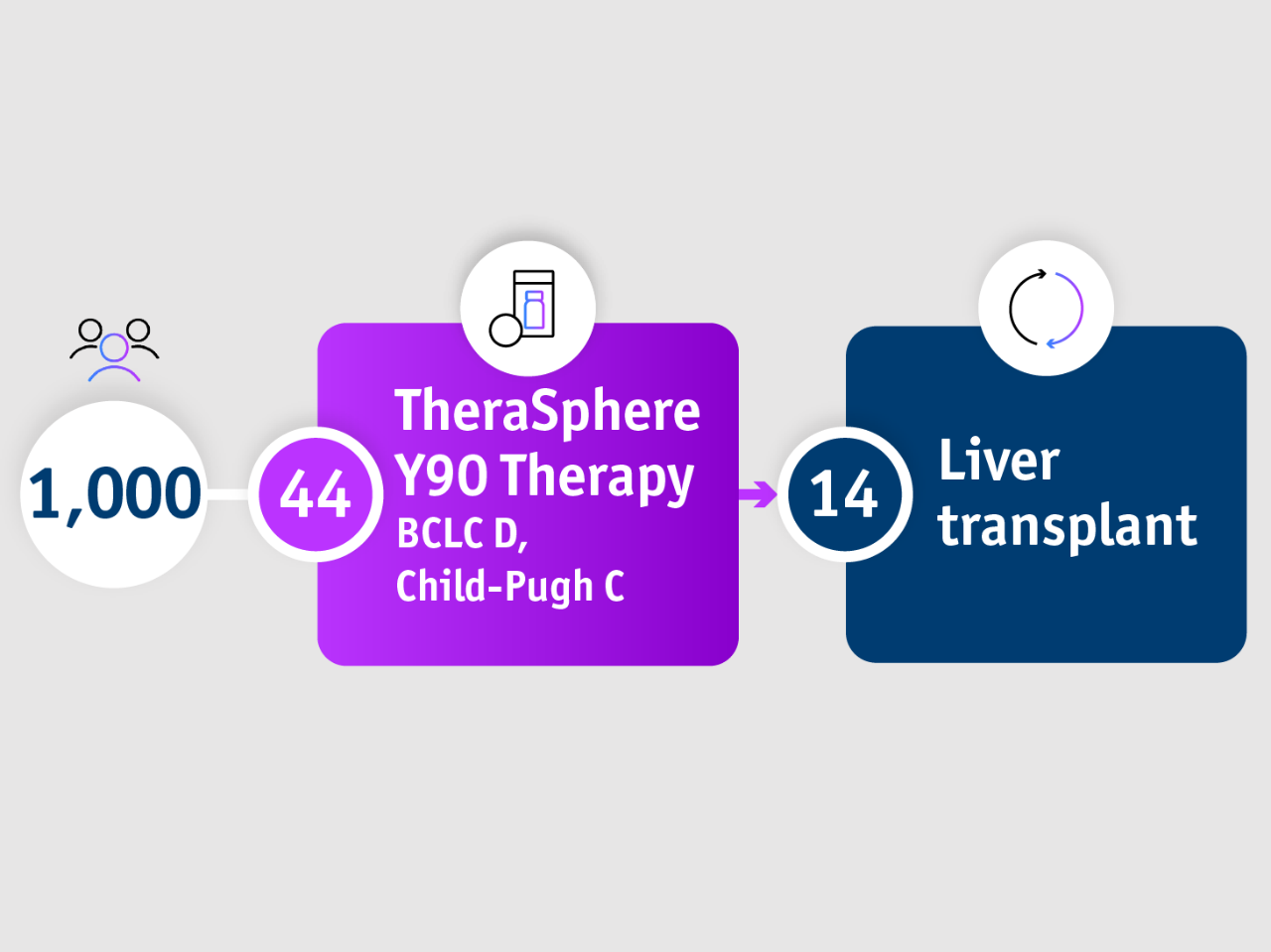
➣ ~1/3 of patients with BCLC D, CP-C stage HCC accessed liver transplantation following TheraSphere Y90 Therapy5
1,000-patient experience: Salem et al. 20185
- Palliative to curative
- Summary
- Study design
Palliative care has traditionally been recommended for BCLC D patients
Salem et al. 2018
• From 2003 to 2017
• 1,000 HCC patients were treated with Y-90 glass microspheres as part of a prospective cohort study (the largest single-centre cohort conducted) with 1,577 total treatments (median: 1, range: 1-8)
Baseline characteristics:
• ECOG: 0 (56%); 1 (40%); 2 (4%)
• Child-Pugh: A (51%); B (45%); C (4%)
• BCLC: A (26%); B (15%); C (54%); D (4%)
No treatment-related deaths
LEGACY: No grade 4 or 5 adverse events (AE) nor deaths were related to treatment, most AEs resolved during the course of the study period, and no patient experienced radiation-induced liver disease.6
Improved tolerability
TheraSphere Y90 Therapy may offer better quality of life.7,8
• A well-tolerated outpatient treatment7
• Safe and effective in the treatment for unresectable HCC, with less post-embolisation syndrome vs chemoembolisation8
• Shorter hospitalisation times, fewer necessitated treatment sessions, and fewer visits to hospital than TACE8
A favourable toxicity profile
TheraSphere Y90 Therapy is safe and effective in the treatment of unresectable HCC and does not prevent patients from receiving subsequent therapies:9,10
• No concern for vascular stasis and less likely to result in vessel spasm and microsphere reflux vs resin SIR-Spheres11
• No gastrointestinal ulcers or pulmonary toxicities reported3,12
• Zero treatment-related deaths and grade 3-4 bilirubin toxicities remained below 14% at 3 months3
*From liver transplant, median OS was 12.5 years (95% CI:120-150).
References
1.Gabr A, al. Liver Transplantation Following Yttrium‐90 Radioembolization: 15‐year Experience in 207‐Patient Cohort. Hepatology 2021;73(3):998-1010.
2.DOSISPHERE-01: Garin E, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021 Jan;6(1):17-29.
3.Mazzaferro V, et al. Yttrium-90 Radioembolization for Intermediate-Advanced Hepatocellular Carcinoma: A Phase 2 Study. Hepatology. 2013;57(5):1826-1837.
4.Ranganathan S, et al. Radioembolization for Intermediate-Stage Hepatocellular Carcinoma Maintains Liver Function and Permits Systemic Therapy at Progression. J Vasc Interv Radiol. 2023;34(6):968-975.
5.Salem R, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68(4):1429-1440.
6.LEGACY: Salem R, et al. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021; 74(5): 2342–2352.
7.Salem R, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497-507.e2.
8.Kim HC. Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23(2):109-114.
9.Kulik LM, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71-81.
10.Mulcahy MF, et al. Radioembolization With Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial. J Clin Oncol. 2021;39(35):3897-3907.
11.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251–1278.
12.Hilgard P, Hamami M, Fouly AE et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52(5):1741–1749.
Abbreviations
AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; LT, liver transplant; OS, overall survival; PVT, portal vein thrombosis; TACE, transarterial chemoembolisation; Y90, yttrium-90.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
TheraSphere is a registered trademark of Theragenics Corporation, used under license by Boston Scientific Medical Device Limited, a wholly owned indirect subsidiary of Boston Scientific Corporation.
* media Overall Survival
CЄ 0123
















