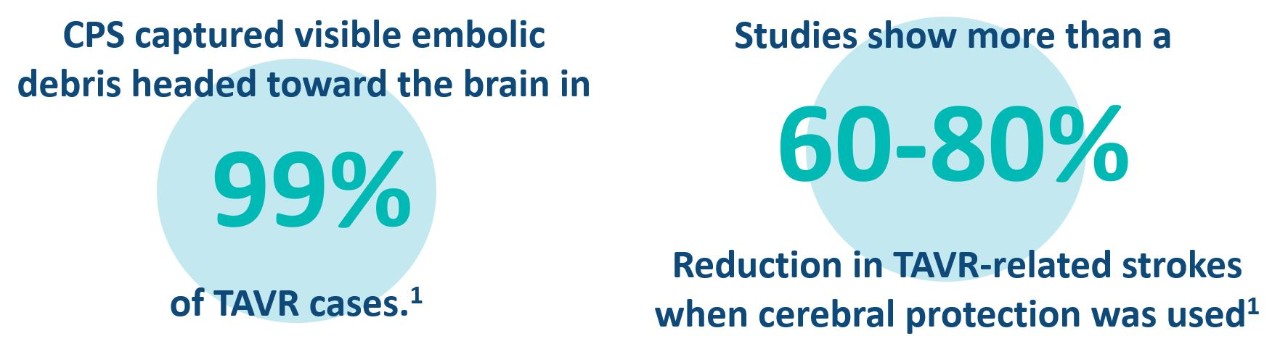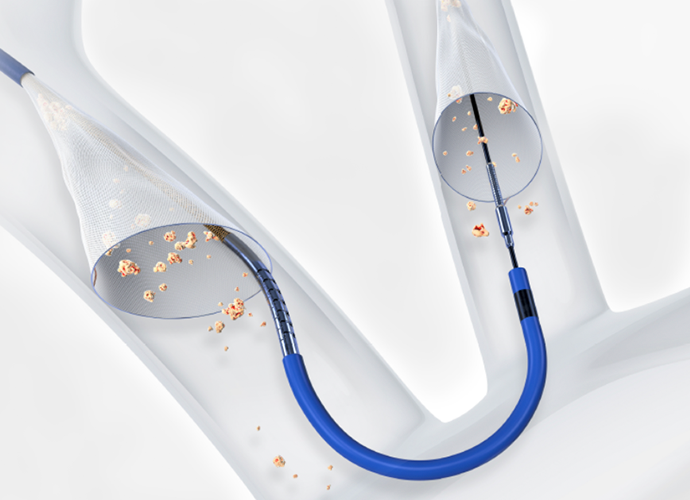

Talk with your doctor about cerebral embolic protection
This guide outlines questions to ask your doctor as you’re considering a TAVR procedure.
Resources
Find frequently asked questions, helpful checklists, informational brochures, and other resources to help you navigate your diagnosis and next steps.
As with any medical device, there are risks associated with use of the SENTINEL Cerebral Protection System. These risks include but are not limited to: infection, blood vessel injury, stroke, death.
Issues specific to the use of the SENTINEL have been reported in a small number of patients and typically went away in 30 days. The use of SENTINEL during yourprocedure is solely at the discretion of your doctor.
1 Source: SENTINEL Clinical Trial
The information presented in this document by Boston Scientific India is for educational purposes only for patient/family member of the patient who is planning to be impl anted with Boston Scientific’s device based on the judgement of Healthcare professional and cannot be circulated further. Healthcare professional holds the responsibility to share the information as deemed appropriate with the patient/family member of the patient. The information should not be treated as comprehensive and does not intend to provide diagnosis, treatment or any medical advice. Responsibility for patient care resides with the healthcare professional on the basis of his or her professional licence, experience and knowledge of the patient. Healthcare professionals must rely on their judgment when deciding which treatments and procedures to use with patients. Individual results may vary and hence, it is advisable to consult your doctor or other qualified health care professional regarding any medical or heal th related diagnosis or treatment options.