SYNERGY™
Everolimus-Eluting Platinum Chromium Coronary Stent System
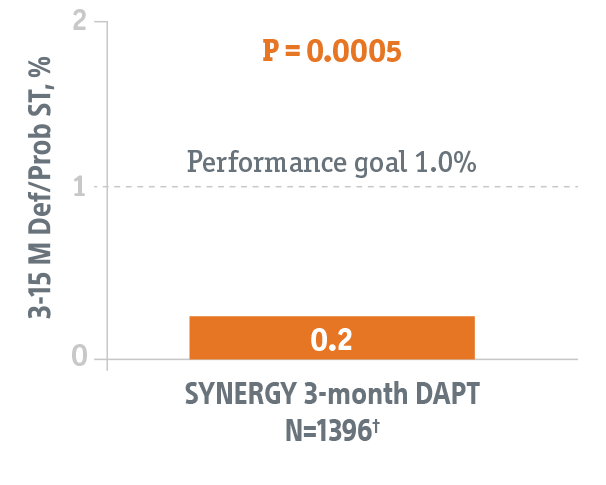
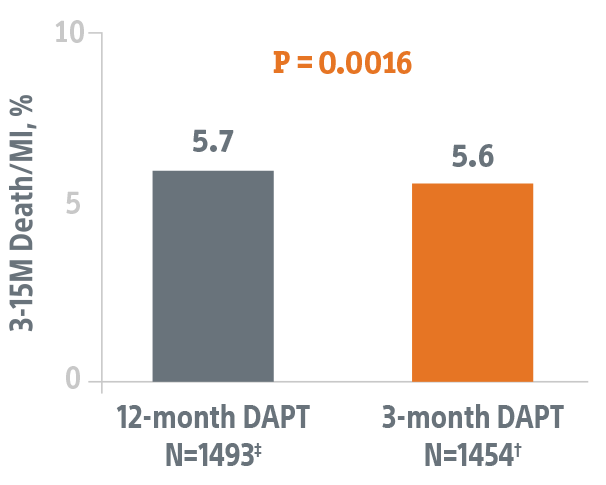
Studies the safety of discontinuing DAPT at 3-months in this HBR patient population using the SYNERGY BP-DES.*
Trial Design
- Prospective, multicenter, single-arm trial
- 2,009 patients with significant bleeding risks at 110 global sites
- Primary endpoint data collected from 1,487 patients eligible to discontinue DAPT at 3-months.