SYNERGY™
Everolimus-Eluting Platinum Chromium Coronary Stent System
How does the polymer on the drug-eluting stent (DES) impact the healing process?
Polymers provide a mechanically stable reservoir for the drug and modulate drug release.1 Once the drug release is complete, the polymer has no remaining function; however, it can still impact the late safety and efficacy of the stent device.
Even the very best permanent polymers are still linked to inflammation, neoatherosclerosis, thrombosis risk, and delayed healing.2 Additionally, 2nd generation DES show a continuous accrual of events of about 2% per year after one year.3 These late event challenges led to the development of the bioabsorbable polymer drug-eluting stent.
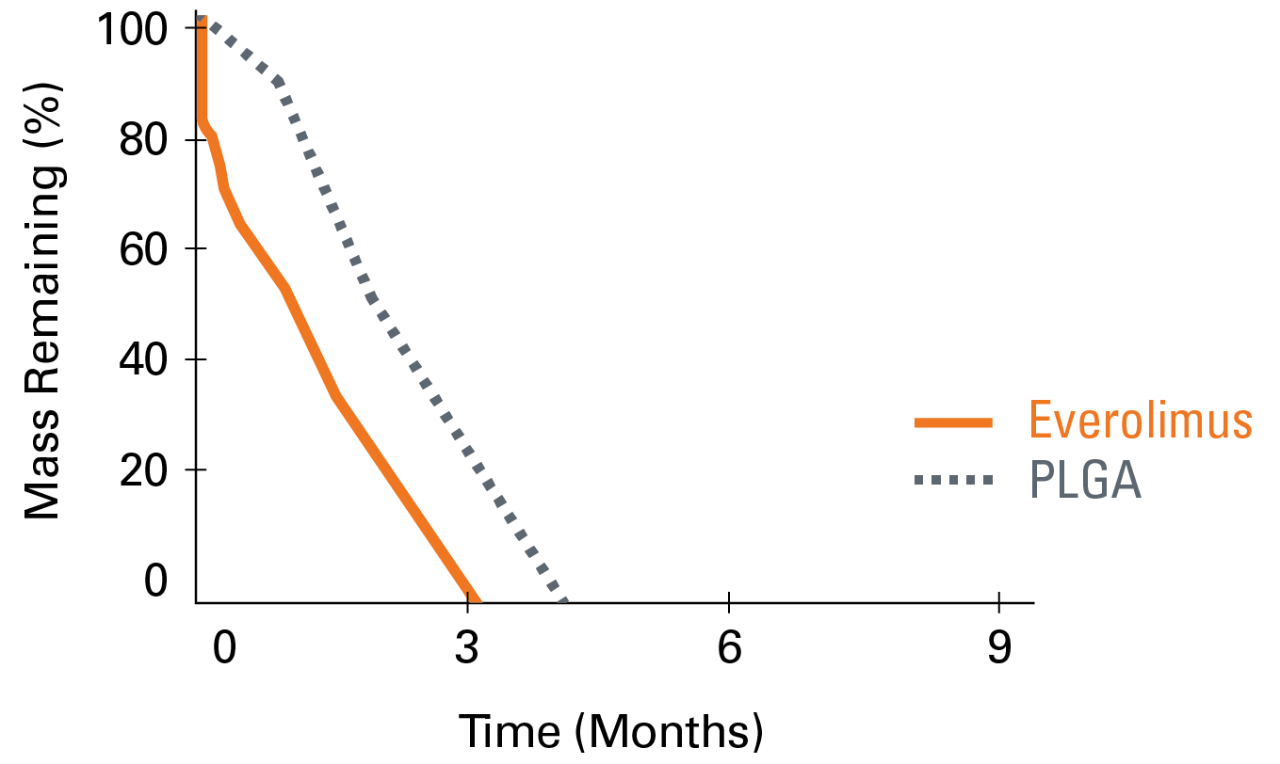
Everolimus is still present in the tissue out to 120-days while polymer completes absorption
1. Parker T, Davé V, Falotico R. Polymers for drug eluting stents. Curr Pharm Des. 2010;16(36):3978-3988.
2. Ernst A, Bulum J. New Generations of Drug-Eluting Stents – A Brief Review. EMJ Int Cardiol. 2014;1:100-106.
3. Adapted from a presentation by Stephan Windecker, MD at PCR 2014.
4. Wilson GJ, Marks A, Berg KJ, et al. The SYNERGY biodegradable polymer everolimus eluting coronary stent: Porcine vascular compatibility and polymer safety study. Catheter Cardiovasc Interv. 2015;86(6):E247-257.
5. Presented by Mike Eppihimer (Boston Scientific), PhD, PCR 2014. Preclinical: Human Coronary Artery Model. Images courtesy of Mike Eppihimer (Boston Scientific), PhD.
In addition to the polymer impact on healing, how does stent architecture play a role and what are the results?
In addition to providing freedom from long-term polymer exposure, the SYNERGY™ BP Stent includes several other design advantages:
- Offset peak-to-peak design with 2 connectors between rings throughout the body of the stent for greater flexibility and conformability
- Platinum chromium metal alloy for increased radial strength, less recoil, and best-in-class visibility
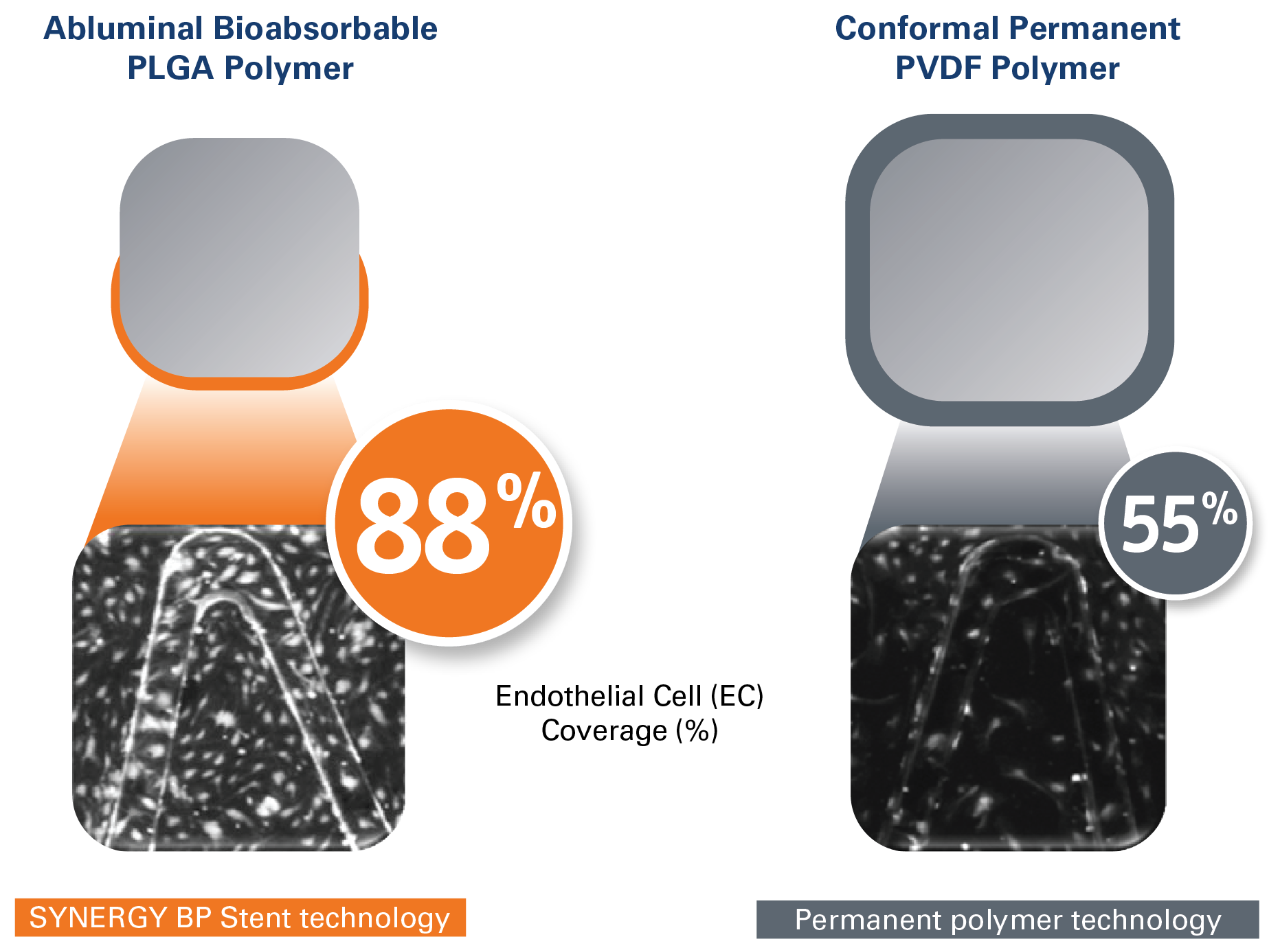
- Thin struts (74 µm)** coated with an ultra-thin abluminal bioabsorbable PLGA polymer, providing:
- Less thrombogenicity due to less disruption of blood flow1
- Faster endothelialization and healing than thicker stent struts2
References:
1. Koppara T, et al. Circ Cardiovasc Int 2015; Modified from Koskinas et al. J Am Coll Cardiol. 2012;59:1337-1349.
2. Soucy N, Feygin J, Tunstall R, et al. Strut tissue coverage and endothelial cell coverage: a comparison between bare metal stent platforms and platinum chromium stents with and without everolimus-eluting coating. EuroIntervention. 2010;6(5):630-637.
** Strut thickness for small vessel model is 0.0029” (74μm), Workhorse model is 0.0031” (79μm) and large vessel is 0.0032” (81μm). Boston Scientific data on file.
What are the guidelines for DAPT duration?
The 2016 ACC/AHA guidelines make the following recommendations regarding the duration of DAPT in patients with stable ischemic heart disease after drug-eluting stent (DES) implantation:1
- Class I recommendation (“should be given”): DAPT for a minimum of 6 months
- Class IIb recommendation (“may be considered”): For those not at high risk for bleeding, continuation beyond 6 months may be reasonable
- Class IIb recommendation (“may be considered”): For those at high risk for bleeding, discontinuation after 3 months may be reasonable
Read the SYNERGY BP Stent Directions for Use for more information on DAPT labeling.
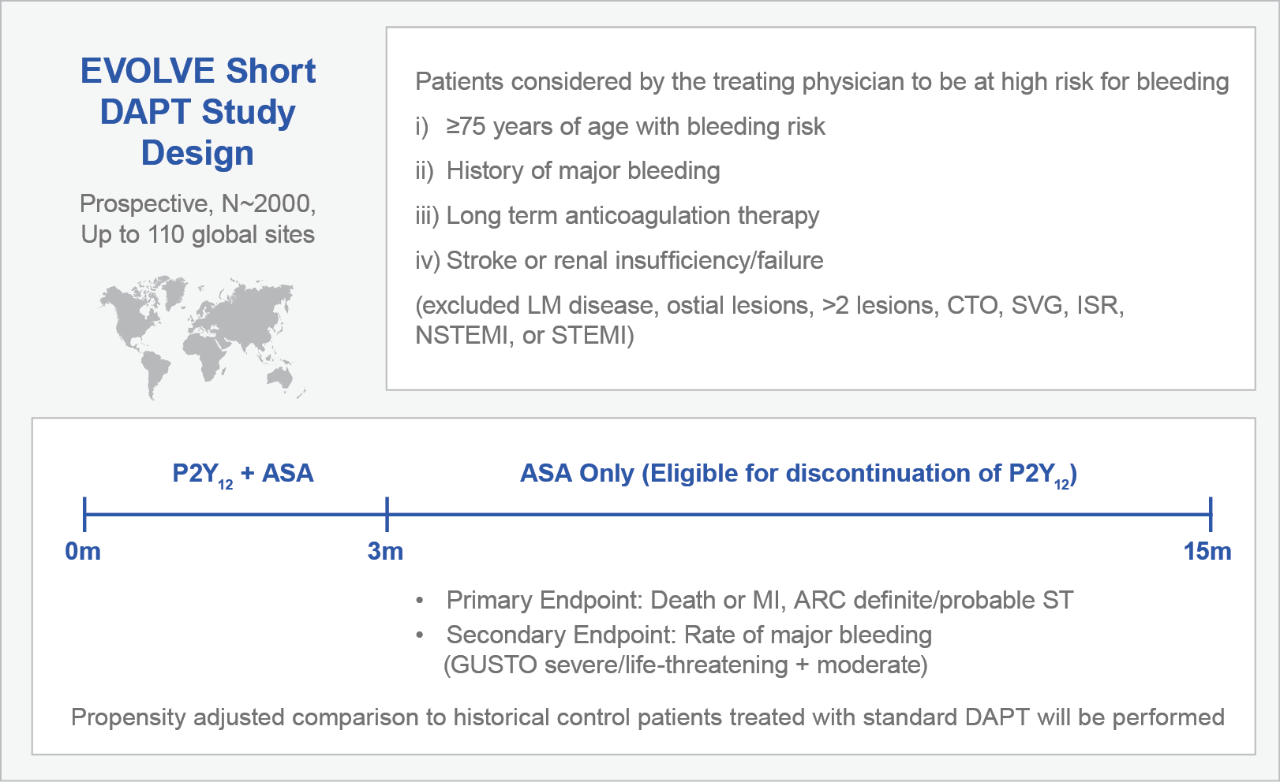
The SYNERGY BP Stent was designed to reduce the risk of late ST compared to permanent polymer DES, as up to 30% of patients discontinue or interrupt DAPT in the first year.2 We are investing in the science to address this significant unmet clinical need. We are the only company running an IDE trial to evaluate a 3-month DAPT regimen in BP-DES patients with a high bleeding risk. Enrollment in the EVOLVE Short DAPT Trial began in February 2016.
References:
1. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016. [Epub ahead of print].
2. Presented by J. Polad , PCR 2013, Effect of Shorter Dual Antiplatelet Therapy on One Year Clinical Outcomes After Implantation of Drug Eluting Stent.
What are the published studies on the SYNERGY BP Stent?
Below you will find links to published studies on the SYNERGY BP Stent. Please use your Pubmed credentials to login and gain access to the articles.
Article: Real-life clinical outcomes with everolimus eluting platinum chromium stent with an abluminal biodegradable polymer in patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR)
Publication: Catheter Cardiovasc Interv., May 2017
Author(s): Sarno G, Lagergvist B, Olivecrona G, et al.
Pubmed Link
Article: Three-month evaluation of strut healing using a novel optical coherence tomography analytical method following bioresorbable polymer everolimus-eluting stent implantation in humans: the TIMELESS study
Publication: Coron Artery Dis., March 2017
Author(s): Vesga B, Hernandez H, Moncada M, et al.
Pubmed Link
Article: Safety and efficacy of a bioabsorbable polymer-coated, everolimus-eluting coronary stent in patients with diabetes: the EVOLVE II diabetes substudy
Publication: Eurointervention., March 2017
Author(s): Kereiakes DJ, Meredith IT, Masotti M, et al.
Pubmed Link
Article: Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial
Publication: Circ Cardiovasc Interv., April 2015
Author(s): Kereiakes DJ, Meredith IT, Windecker S, et al.
Pubmed Link
Article: Six-month IVUS and two-year clinical outcomes in the EVOLVE FHU trial: a randomised evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting stent
Publication: Eurointervention. May 2013
Author(s): Meredith IT, Verheye S, Weissman NJ, et al.
Pubmed Link
Article: Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent
Publication: J Am Coll Cardiol., April 2012
Author(s): Meredith IT, Verheye S, Dubois CL, et al.
Pubmed Link