Detachable Coils Portolio
Solutions for FRAMING, FILLING and FINISHING in Peripheral Embolization
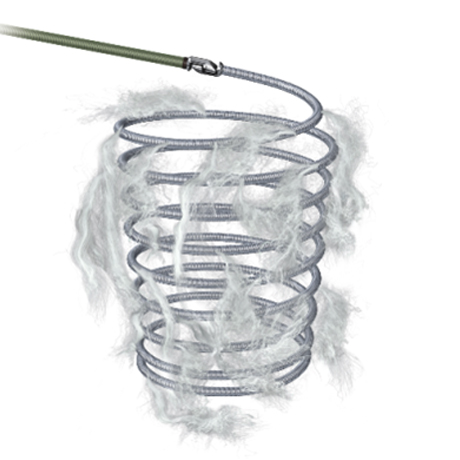
Associates Precision & Control of a detachable coil to the powerful Thrombogenicity of a fibered coil configuration. The Interlocking arm is designed to allow the coil to be advanced and retracted before final placement. Synthetic streamlined oriented & dense fibers onto the platinum coil to foster stasis and help reaching occlusion. Available in a broad range of product sizes, Interlock™ Fibered occlusion system offers precise treatment and clinical flexibility.
Product details
Shapes
Fibered for Thrombogenicity Power
- Every Coil contains multiple dense networks of Dacron® Fibers, each designed to catch blood cells and propagate the formation of thrombus.
- A close-up image of Dacron Fibers (left) shows that the structure of the fiber strands are robust, yet porous, allowing for rapid thrombosis.
Catheter Compatibility:
Physicians should exercise their clinical judgment in selection and use of catheters. Refer to the instructions for use for each product listed. Manufacturers may make changes to their catheters without notice which may impact their suitability for use with Interlock - 35 Fibered IDC Occlusion System. Boston Scientific Corporation provides no warranty for use of third party catheters with its products. The use of other diagnostic catheters may result in an inability to deliver, deploy, or recapture the device.
Do not attempt to use the Interlock - 35 Coil with a soft-walled delivery catheter, such as the Terumo Glidecath™ Catheter or the Angiodynamics SoftVu™ Catheter. Due to the coil’s high radial force and dense fibering configuration, significant resistance will be encountered when advancement through a soft-walled delivery catheter is attempted.
Coil Preparation
Ordering Information:
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001363500 | Interlock - 35 Coil | 3 | 40 | 2D |
| M001363520 | Interlock - 35 Coil | 4 | 100 | 2D |
| M001363540 | Interlock - 35 Coil | 6 | 100 | 2D |
| M001363550 | Interlock - 35 Coil | 6 | 200 | 2D |
| M001363570 | Interlock - 35 Coil | 8 | 100 | 2D |
| M001363580 | Interlock - 35 Coil | 8 | 200 | 2D |
| M001363590 | Interlock - 35 Coil | 8 | 400 | 2D |
| M001363600 | Interlock - 35 Coil | 10 | 200 | 2D |
| M001363610 | Interlock - 35 Coil | 10 | 400 | 2D |
| M001363620 | Interlock - 35 Coil | 12 | 200 | 2D |
| M001363630 | Interlock - 35 Coil | 12 | 400 | 2D |
| M001363640 | Interlock - 35 Coil | 15 | 200 | 2D |
| M001363650 | Interlock - 35 Coil | 15 | 400 | 2D |
| M001363660 | Interlock - 35 Coil | 18 | 200 | 2D |
| M001363670 | Interlock - 35 Coil | 18 | 400 | 2D |
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001363700 | Interlock - 35 Coil | 4 | 60 | Cube |
| M001363720 | Interlock - 35 Coil | 6 | 100 | Cube |
| M001363730 | Interlock - 35 Coil | 6 | 200 | Cube |
| M001363760 | Interlock - 35 Coil | 8 | 200 | Cube |
| M001363790 | Interlock - 35 Coil | 10 | 250 | Cube |
| M001363800 | Interlock - 35 Coil | 10 | 400 | Cube |
| M001363810 | Interlock - 35 Coil | 15 | 250 | Cube |
| M001363820 | Interlock - 35 Coil | 15 | 400 | Cube |
| M001363830 | Interlock - 35 Coil | 20 | 400 | Cube |
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001363910 | Interlock - 35 Coil | 4 | 45 | Diamond |
| M001363920 | Interlock - 35 Coil | 6 | 90 | Diamond |
| M001363930 | Interlock - 35 Coil | 8 | 140 | Diamond |