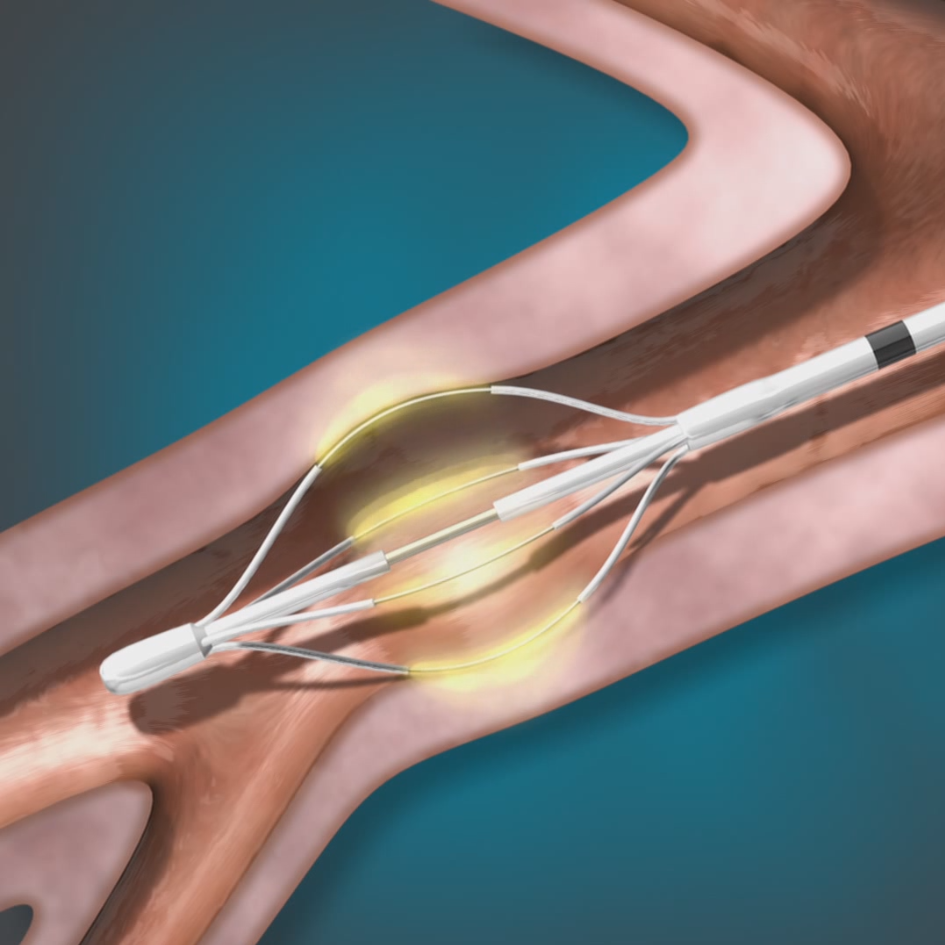
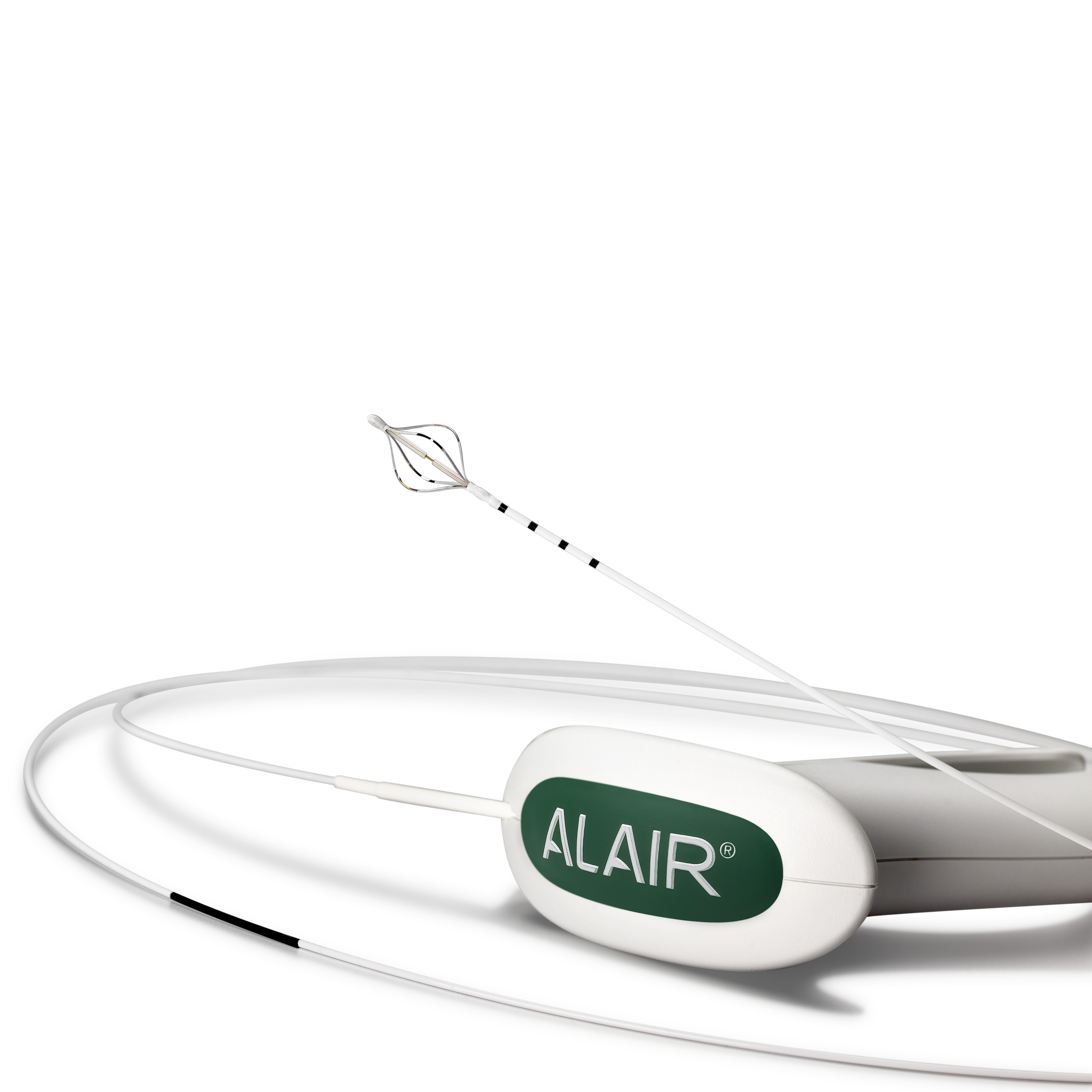
The Alair™ Bronchial Thermoplasty System
Bronchial Thermoplasty (BT), delivered by the Alair™ System, is a safe outpatient procedure for adult patients with severe asthma.
Key Resources
Explore
Product Details
Ordering Information
Alair™ Bronchial Thermoplasty System* |
|||
|---|---|---|---|
| Order Number | Model Number | Description | Packaging |
| M005ATS20000 | Alair ATS 200 | ALAIR BT CONTROLLER |
Each |
| M005ATS25010 | Alair ATS 2-5 | Alair BT Catheter North America |
Each |
| M005ATS20110 | Alair ATS 201 | ALAIR ACCESSORY KIT -NORTH AMERICAN PLUG |
Each |
*Service Agreement available with 1, 2, 3, and 4 year terms. **Note: Initial stocking order requires a minimum order of 6 catheters (covering the complete treatment of 2 patients). |
|||