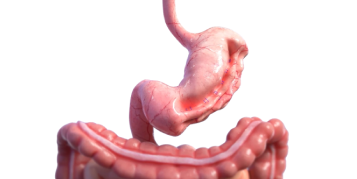
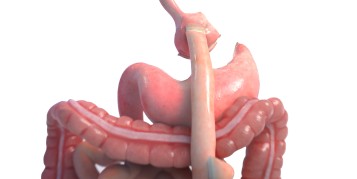
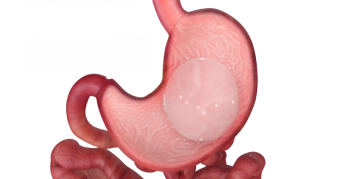
Introducing Endura Weight Loss Solutions
Patients living with obesity face many challenges on their weight loss journey – from making diet, exercise, and lifestyle changes to managing the ups and downs of their physical, mental, and emotional health. It’s a journey that requires real endurance.
Boston Scientific understands these challenges for your patients, and we offer a new approach to endobariatrics that provides innovative endoscopic options for physicians. Minimally invasive procedures. Proven, effective results. Endura Weight Loss Solutions. The future of weight loss is here.
1. Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. Lancet. 2022;400:441–451.
2. Jirapinyo P, Kumar N, AlSamman MA, Thompson CC. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest Endosc. 2019;91:1067–1073.
3. US FDA Pivotal Study. n = 137 Orbera, n = 136 control, p < 0.001. Most of the patients from the US FDA pivotal study were female and Caucasian. Because of this, data from this study may not accurately represent the same effectiveness and safety profile in Hispanic, African American, or other ethnic populations.
Learn more about Endura Weight Loss Solutions
Download the Endura Weight Loss Solutions Frequently Asked Questions guide to learn more about this category of minimally invasive weight loss options for your patients. Get answers to common questions, patient insights, and training info—all in one place.
Caution: The information presented in this document by Boston Scientific is intended for healthcare professionals in India. [The information presented in this document is intended to provide balanced, scientific, and evidence based answers to unsolicited medical questions.] The information should not be, however, treated as comprehensive and does not intend to provide diagnosis, treatment or any medical or health advice. For full information about the product, please refer to the appropriate product labeling. Responsibility for patient care resides with the healthcare professional on the basis of his or her professional license, experience and knowledge of the patient. Healthcare professionals must rely on their judgment when deciding which treatments and procedures to use with patients.
As a condition of the FDA De Novo Authorization of the Overstitch NXT and Overstitch Endoscopic Suturing System for endobariatric procedures (formerly referred to as the Apollo ESG and Apollo REVISE Systems), the devices should only be used for Endoscopic Sleeve Gastroplasty (ESG) or to enable transoral outlet reduction (TORe) as a bariatric revision procedure by gastroenterologists and surgeons who have undergone specific training by the device manufacturer.
To fulfill the FDA requirement and special controls for these devices, Boston Scientific is required to independently host courses with consistent training curricula. More information regarding the referenced ESG and TORe revision procedure training courses is available through Boston Scientific.