Thank you for your interest in the OPTION Trial. This trial is active and has reached full enrollment.
OPTION - AFib, Ablation, and Stroke - Boston Scientific
What Is AFib?
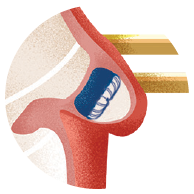
Atrial Fibrillation (AFib) is a heart condition in which the upper chambers of your heart quiver. This condition can cause blood clots to form in an area of your heart called the left atrial appendage (LAA). If a blood clot forms here, it can travel through an artery to the brain and cause a stroke.
People with AFib have a five-times greater risk for stroke than people with normal heart rhythms.1
Managing AFib and Stroke Risk
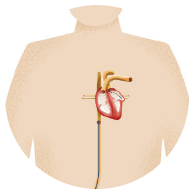
While completely curing AFib may not be possible, medications and/or treatments such as an AFib ablation, a procedure to burn or freeze the tissue around the veins in the upper left chamber of your heart, can help decrease the burden of AFib. However, even after an ablation, you are still at risk of having a stroke.
Blood thinners or Left Atrial Appendage Closure (LAAC) implants like WATCHMAN address the need for stroke reduction in AFib patients.
WATCHMAN is a one-time procedure that does not require open heart surgery and may reduce stroke risk for a lifetime. The device is about the size of a quarter, and eliminates the need for long-term blood thinner use. It is implanted into the left atrial appendage of your heart to permanently close off this small pouch and keep harmful blood clots from escaping and causing a stroke.