Defibrillators (ICD)
All Specialties (-)
-
DYNAGEN™ MINI ICD The world’s smallest and thinnest ICD.1
Boston Scientific provides devices tailored to individual needs. Up to 20% smaller and 24% thinner than some ICDs on the market,2 the MINI offers all the power of a Boston Scientific ICD in a device that is the industry’s sleekest and designed for patient comfort.
- Electrophysiology
-
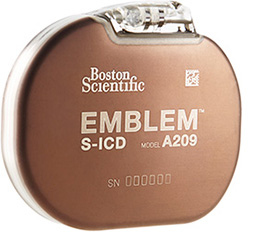
EMBLEM™ MRI S-ICD System Subcutaneous Implantable Defibrillator
The Only Device of its Kind
The EMBLEM MRI S-ICD is the only subcutaneous implantable defibrillator system that provides protection from both sudden cardiac death and the risks and complications associated with transvenous leads.
- Electrophysiology
-
INOGEN™ MINI ICD The world’s smallest and thinnest ICD.1
Boston Scientific provides devices tailored to individual needs. Up to 20% smaller and 24% thinner than some other ICDs on the market,2 the MINI offers all the power of a Boston Scientific ICD in a device that is the industry’s sleekest and designed for patient comfort.
- Electrophysiology
-
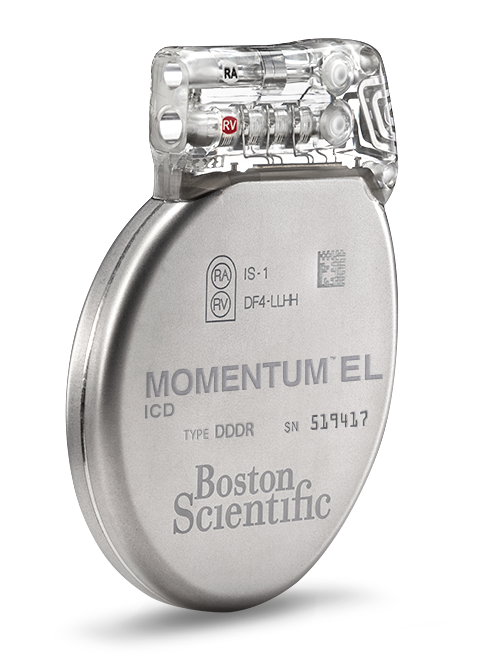
MOMENTUM™ EL (Extended Longevity ICD) Powered by EnduraLife™ Battery Technology
Models D120 and D121The MOMENTUMTM EL (Extended Longevity) ICD offers an extraordinary, holistic approach to cardiac care. In addition to the HeartLogicTM Heart Failure Diagnostic and ImageReadyTM MR-Conditional technology, MOMENTUM EL features EnduraLifeTM Battery Technology which provides the clinical freedom to make programming decisions without worrying about the battery.
- Electrophysiology
-
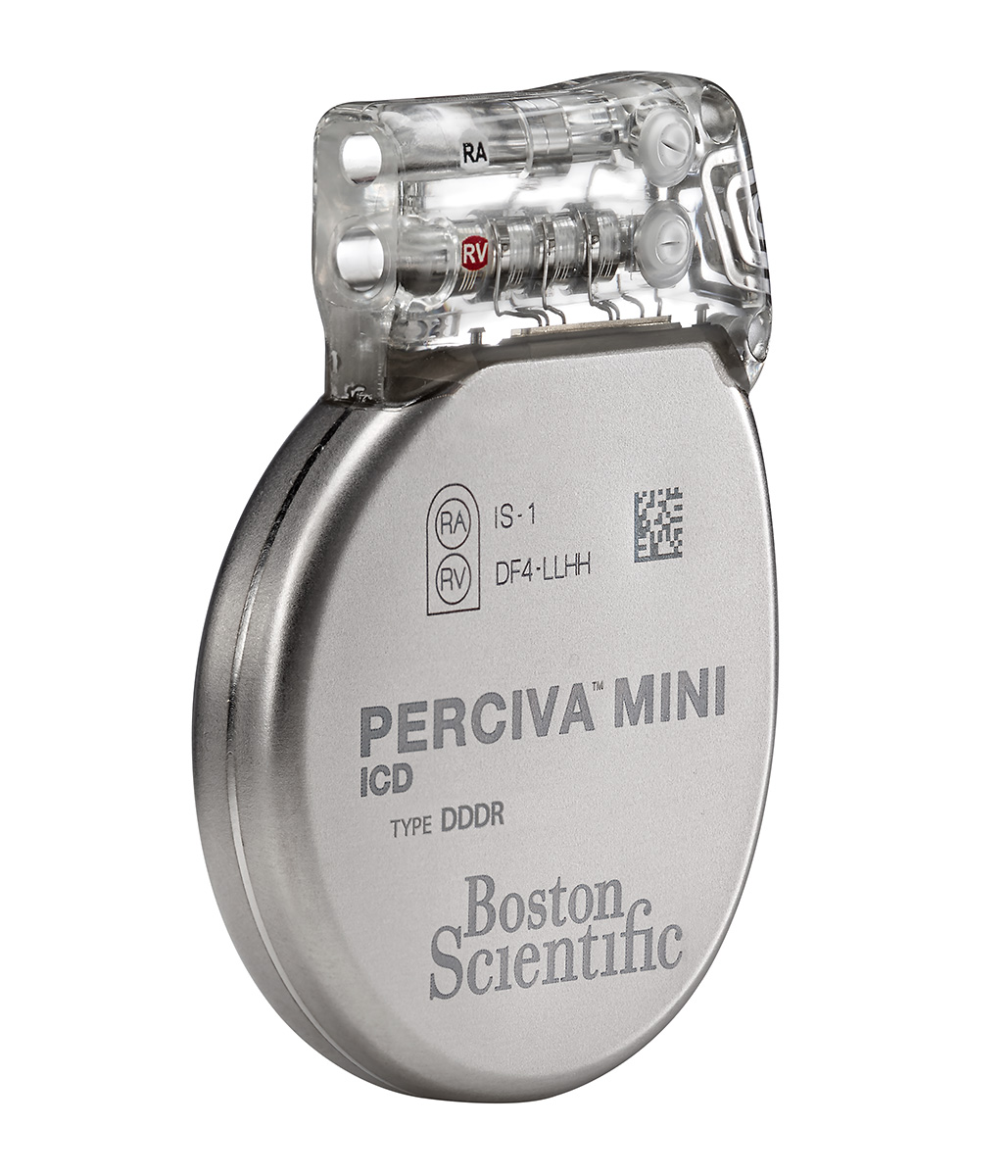
PERCIVA™ ICD Models D400, D401, D412, D413
The PERCIVATM ICD is the industry's smallest ICD and features exclusive technologies such as HeartLogicTM Heart Failure Diagnostic and an ImageReadyTM MR-Conditional system.
- Electrophysiology
-
RESONATE™ EL ICD Powered by EnduraLife™ Battery Technology
Models D432 and D433The RESONATETM EL (Extended Longevity) ICD offers an extraordinary, holistic approach to cardiac care. In addition to the HeartLogicTM Heart Failure Diagnostic and ImageReadyTM MR-Conditional technology, RESONATE EL features EnduraLifeTM Battery Technology which provides the clinical freedom to make programming decisions without worrying about the battery.
- Electrophysiology
-
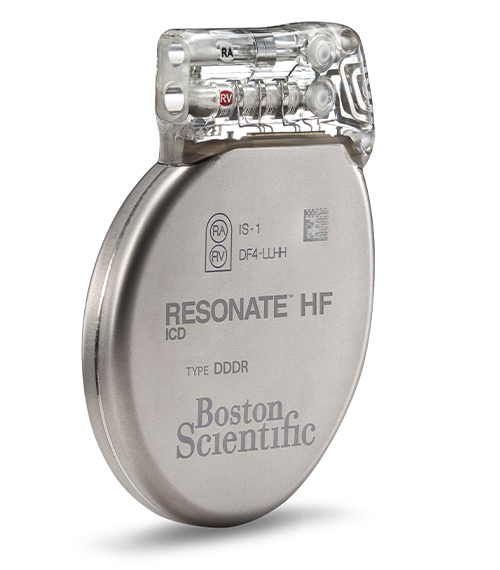
RESONATE™ HF ICD Powered by EnduraLife™ Battery Technology
Model D532, D533Our most comprehensive technology for implantable heart failure management, RESONATE™ HF, features the only physiologic sensor proven to restore chronotropic competence1 by adjusting patient heart rate according to changes in movement and respiration. Equipped with a suite of industry-leading HF technologies (RightRate™ and HeartLogic™) plus Boston Scientific's exclusive EnduraLife™ Battery Technology, RESONATE HF ICD provides physicians with an unprecedented level of insight, power and control.
- Electrophysiology
-
VIGILANT™ EL ICD Powered by EnduraLife™ Battery Technology
Models D232 and D233The VIGILANTTM EL (Extended Longevity) ICD features EnduraLifeTM Battery Technology in addition to the HeartLogicTM Heart Failure Diagnostic and ImageReadyTM MR-Conditional technology to help manage patient comorbidities.
- Electrophysiology