EMERGE™
PTCA Dilatation Catheter
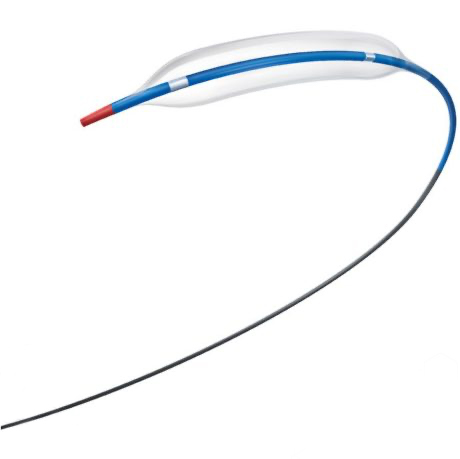
EMERGE is a predilatation balloon catheter designed to navigate and cross even the most challenging lesions with ease. With exceptional deliverability, an ultra-low tip profile, and unparalleled expansion range, it offers complete support for vessels of any size and complexity.
Explore
Product Details
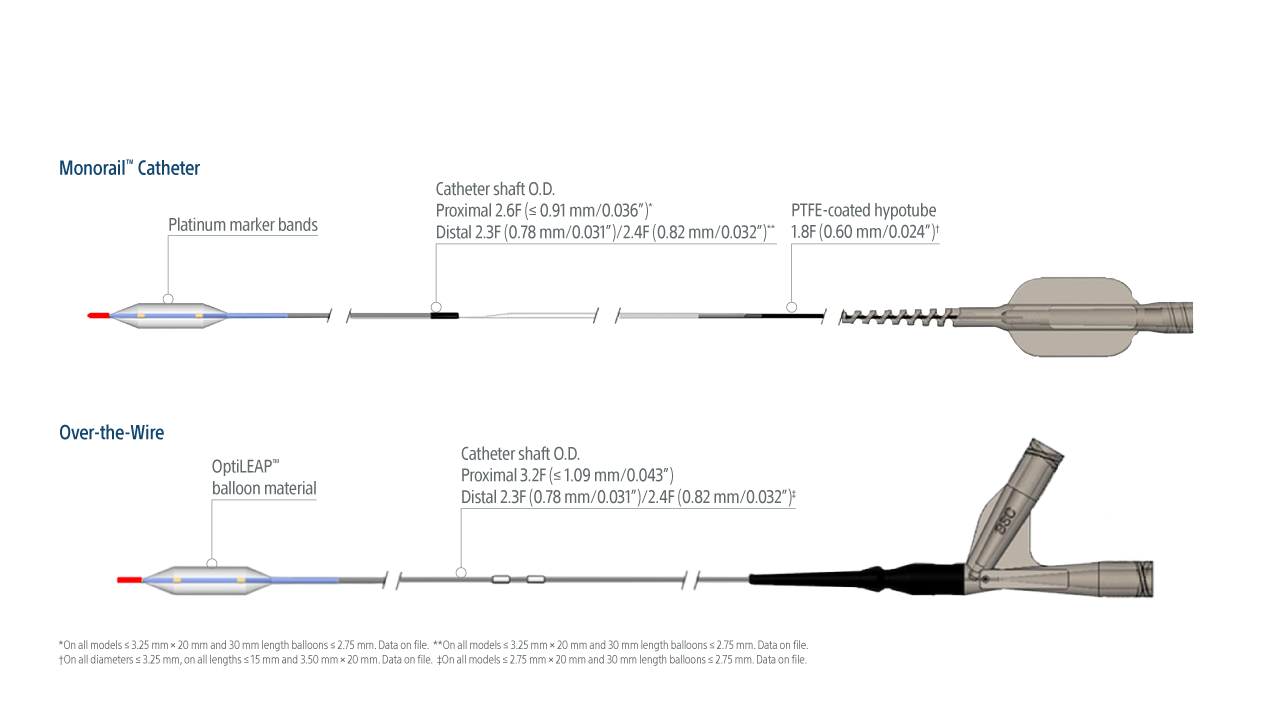
*On all models ≤3.25 mm x 20 mm and 30 mm length balloons ≤2.75 mm. Data on file. ** On all models ≤3.25 mm x 20 mm and 30 mm length balloons ≤2.75 mm.
Data on file.
†On all diameters ≤3.25 mm, on all lengths ≤15 mm and 3.50 mm x 20 mm. Data on file. ‡On all models ≤2.75 mm x 20 mm and 30 mm length balloons ≤2.25 mm.
Data on file.
Small 1.2 mm Size
- Exceptional deliverability and low profiles designed to cross tight lesions
- High rated burst pressure 18 ATM (1824 kPa) for sizing flexibility
- Two shaft designs provide options for challenging lesions
Reduced Shaft Profile for Simultaneous Use
- EMERGE Catheter is designed for exceptional simultaneous use performance
- Shaft profile allows for simultaneous use of two Monorail™ catheters in a 6 F guide catheter and two Over-the-Wire catheters in an 8 F guide catheter*
*6 F guide catheter with a minimum 0.070” ID, 8 F guide catheter with a minimum 0.088” ID
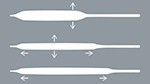
Unparalleled Expansion Range
- Designed to optimize stent apposition in large, proximal vessels
- Available in 4.50 and 5.00 mm diameters
- Extended sizes now match 40 mm length
New Hydrophilic Coating
- EMERGE New Hydrophilic Coating
- ZGlide™ hydrophilic coating reduces frictional force on the catheter shaft by 51% in bench tests†
†Testing completed on 2.5 x 15 mm Emerge product (n = 18) and 2.5 x 20 mm Apex product (n = 14). Testing completed by Boston Scientific Corporation. Bench test results may not necessarily be indicative of clinical performance. Data on File.
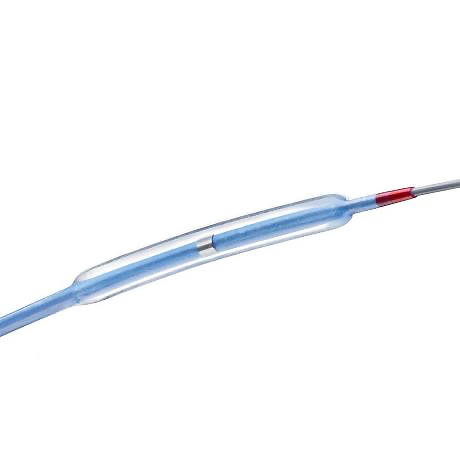
Balloon and Tip Design
- Unique, over-the-inner tip design: outer tip material rides over the inner shaft material and is designed to improve overall flexibility and tip performance
- Profiles: Ultra-low 0.017” tip profile and 0.026” crossing profile‡
- Balloon Material: OptiLEAP™ balloon material provides sizing flexibility
- Platinum marker bands provide optimal radiopacity
‡Crossing profile is defined as the maximum diameter found between the proximal end of the balloon and the distal tip of the catheter. Definition excerpted from FDA Guidance document titled, Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheters. Emerge 0.026” crossing profile measured on 1.2 x 15 mm (n=5) and 1.5 x 15 mm (n = 5) products.
Testing completed by Boston Scientific Corporation. Bench test results may not necessarily be indicative of clinical performance. Data on file.
Dual shaft designs
- Two shaft options with distinct technologies designed to provide flexibility for navigating to and through even the most challenging lesions
- Push technology: Single-segment inner shaft design for ultimate pushability 1.2 mm and 1.5 mm Push
- Workhorse technology: Bi-Segment™ inner shaft designed for maximum deliverability without sacrificing pushability (1.2 mm to 4.0 mm)
Monorail and Over-the-Wire Catheter Options
Ordering Information
EMERGE Monorail
| MR | Balloon Length | |||||
| Balloon Diameter (mm) | 8 mm | 12 mm | 15 mm | 20 mm | 30 mm | 40 mm |
| 1.20 mm | H7493918908120 | H7493918912120 | H7493918915120 | H7493918920120 | ||
| 1.50 mm | H7493918908150 | H7493918912150 | H7493918915150 | H7493918920150 | ||
| 2.00 mm | H7493918908200 | H7493918912200 | H7493918915200 | H7493918920200 | H7493918930200 | H7493918940200 |
| 2.25 mm | H7493918908220 | H7493918912220 | H7493918915220 | H7493918920220 | H7493918930220 | |
| 2.50 mm | H7493918908250 | H7493918912250 | H7493918915250 | H7493918920250 | H7493918930250 | H7493918940250 |
| 2.75 mm | H7493918908270 | H7493918912270 | H7493918915270 | H7493918920270 | H7493918930270 | |
| 3.00 mm | H7493918908300 | H7493918912300 | H7493918915300 | H7493918920300 | H7493918930300 | H7493918940300 |
| 3.25 mm | H7493918908320 | H7493918912320 | H7493918915320 | H7493918920320 | H7493918930320 | |
| 3.50 mm | H7493918908350 | H7493918912350 | H7493918915350 | H7493918920350 | H7493918930350 | H7493918940350 |
| 3.75 mm | H7493918908370 | H7493918912370 | H7493918915370 | H7493918920370 | H7493918930370 | |
| 4.00 mm | H7493918908400 | H7493918912400 | H7493918915400 | H7493918920400 | H7493918930400 | H7493918940400 |
| 4.50 mm | H7493918908450 | H7493918912450 | H7493918915450 | H7493918920450 | ||
| 5.00 mm | H7493918908500 | H7493918912500 | H7493918915500 | H7493918920500 | ||
EMERGE OTW
OTW | Balloon Length | ||||
Balloon Diameter (mm) | 8mm | 12mm | 15mm | 20mm | 30mm |
| 1.20 mm | H7493919108120 | H7493919112120 | H7493919115120 | H7493919120120 | |
| 1.50 mm | H7493919108150 | H7493919112150 | H7493919115150 | H7493919120150 | |
| 2.00 mm | H7493919108200 | H7493919112200 | H7493919115200 | H7493919120200 | H7493919130200 |
| 2.25 mm | H7493919108220 | H7493919112220 | H7493919115220 | H7493919120220 | H7493919130220 |
| 2.50 mm | H7493919108250 | H7493919112250 | H7493919115250 | H7493919120250 | H7493919130250 |
| 2.75 mm | H7493919108270 | H7493919112270 | H7493919115270 | H7493919120270 | H7493919130270 |
| 3.00 mm | H7493919108300 | H7493919112300 | H7493919115300 | H7493919120300 | H7493919130300 |
| 3.25 mm | H7493919108320 | H7493919112320 | H7493919115320 | H7493919120320 | H7493919130320 |
| 3.50 mm | H7493919108350 | H7493919112350 | H7493919115350 | H7493919120350 | H7493919130350 |
| 3.75 mm | H7493919108370 | H7493919112370 | H7493919115370 | H7493919120370 | H7493919130370 |
| 4.00 mm | H7493919108400 | H7493919112400 | H7493919115400 | H7493919120400 | H7493919130400 |
EMERGE Push
MR | Balloon Length | |||
Balloon Diameter (mm) | 8mm | 12mm | 15mm | 20mm |
1.20 Push | H7493919008120 | H7493919012120 | H7493919015120 | H7493919020120 |
1.50 Push | H7493919008150 | H7493919012150 | H7493919015150 | H7493919020150 |
OTW | Balloon Length | |||
Balloon Diameter (mm) | 8mm | 12mm | 15mm | 20mm |
1.20 Push | H7493919208120 | H7493919212120 | H7493919215120 | H7493919220120 |
1.50 Push | H7493919208150 | H7493919212150 | H7493919215150 | H7493919220150 |
Reimbursement
The C-Code used for EMERGE PTCA Dilatation Catheter is C1725 Catheter, Transluminal, Angioplasty, Non-Laser (may include guidance, infusion/perfusion capability). C-Codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific Corporation is not responsible for correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.