If you are not implanted with a cardiac monitoring system, please visit the cardiac monitoring procedure page for more information. View this content in Spanish.
Boston Scientific accounts are for healthcare professionals only.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care
If you are not implanted with a cardiac monitoring system, please visit the cardiac monitoring procedure page for more information. View this content in Spanish.
Recovery from the insertion procedure should not prevent you from returning to an active lifestyle. Your health care provider will help you decide what level of activity is best for you and answer any questions you have about living with your LUX-Dx™ Insertable Cardiac Monitor (ICM) System. Follow your health care provider’s post-operative instructions.
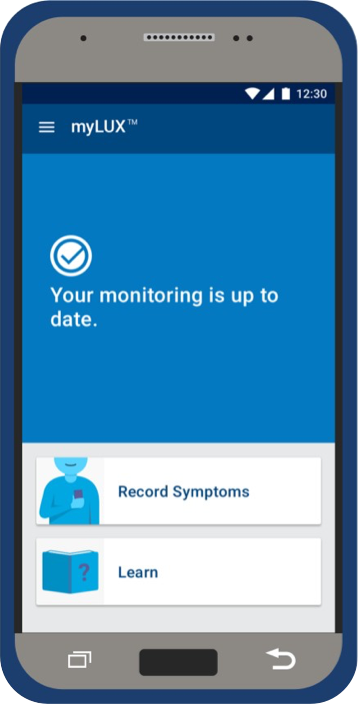
If you see a blue screen, that means your devices are connected and your monitoring is up-to-date.
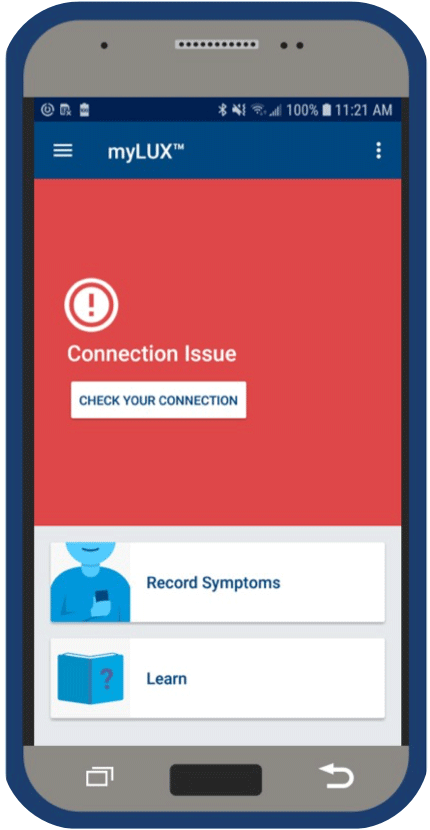
If your myLUX™ app can’t connect to your ICM device or to a cellular or Wi-Fi network, a message will appear on the screen telling you to check your connection. Tap the button displayed with the connection message and follow the instructions until you see a screen confirming your monitor is up-to-date.
The myLUX app Patient Handbook and Quick Start Guide should help you get started. Or, watch a quick video for step-by-step instructions on how to set up your app. If you need additional help, give us a call at 1-866-484-3268.
Yes.
Yes. It is free at no extra cost to you or your clinic. It is exclusively offered to patients who choose to download the myLUX app on their personal smartphones and sign up for the service. The RhythmCARE Assist service is different from remote monitoring services provided by your clinic. Read the RhythmCARE Assist System Service Guide for more details.
Talk to your health care provider about the potential charges related to remote monitoring of your heart.
Our RhythmCARE™ Patient Services team is here to support you throughout your journey.
LUX-Dx ICM System refers to LUX-Dx™, LUX-Dx II™, and LUX-Dx II+™ ICM Systems.
The downloadable myLUX patient app is only available for LUX-Dx II and LUX-Dx II+ ICM. It is not available on LUX-Dx ICM.
Some pictures of phone screens may look different from the screen on your mobile device, based on specific phone models or OS versions.
All trademarks are the property of their respective owners.