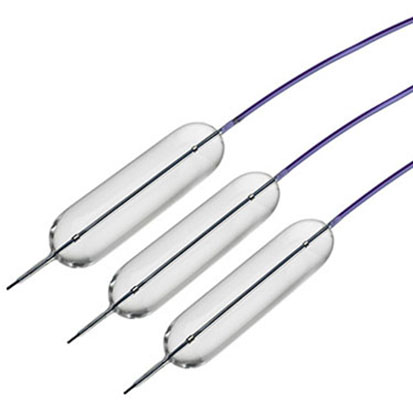
Gallbladder drainage with Lumen Apposing Metal Stent
A non-surgical treatment option for patients at high risk or unsuitable for surgery.
A reliable tool for gallbladder drainage
In most cases, early laparoscopic cholecystectomy is considered the treatment of choice for acute cholecystitis. However, in patients who are elderly, critically ill, or with significant comorbidities, cholecystectomy is considered a high-risk procedure and gallbladder drainage (GBD) is recommended as an alternative treatment.1,2
Of note, the ESGE recommends that in patients at high surgical risk, EUS-guided GBD should be favored over percutaneous gallbladder drainage where both techniques are available, owing to the lower rates of adverse events and need for re-interventions with EUS-GBD.2
The Hot AXIOS™ Stent and Electrocautery-Enhanced Delivery System is an additional treatment option for patients at high risk or unsuitable for surgery - specifically designed and indicated for the drainage of the biliary tract. An established evidence base has demonstrated its clinical and technical success in creating a new temporary opening between the gallbladder and GI tract for symptomatic cholecystitis patients at high risk or unsuitable for surgery. EUS-GBD using Hot AXIOS™ is a ground-breaking endoscopic option in high-risk surgical patients with acute cholecystitis when performed by an experienced endoscopist.3
The Hot AXIOSTM Stent and Electrocautery-Enhanced Delivery System is indicated for use to facilitate transgastric or transduodenal endoscopic drainage of:
- A pancreatic pseudocyst or a walled-off necrosis with ≥70% fluid content
- The gallbladder in patients with acute cholecystitis who are at high risk or unsuitable for surgery
- The bile duct after failed ERCP in patients with biliary obstruction due to a malignant stricture
Recommended stent selection method
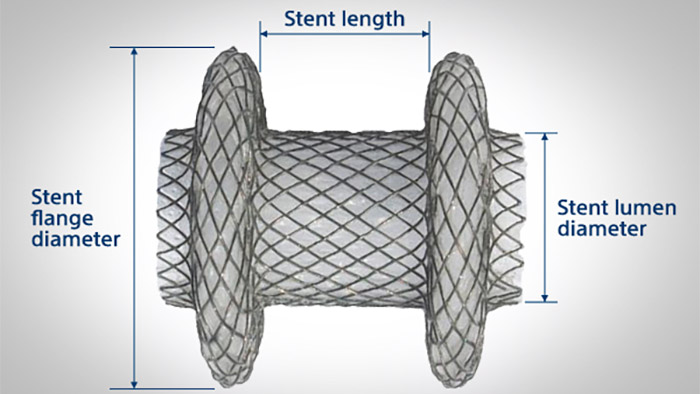
NOTE: Ensure there is sufficient space within the target structure to accommodate the Hot AXIOSTM stent flange diameter.
Gallbladder – Select a stent lumen diameter 10 mm or 15 mm based on gallbladder content via EUS imaging.
WARNING: The safety and effectiveness of the 20 mm stent for drainage of the gallbladder and bile duct has not been established.
The stent length (8 mm or 10 mm) is selected to accommodate the combined tissue wall thickness.
Refer to device Instructions for Use for additional Contraindications, Warnings and Precautions.
View product information for Hot AXIOSTM Stent and Electrocautery Enhanced Delivery System:
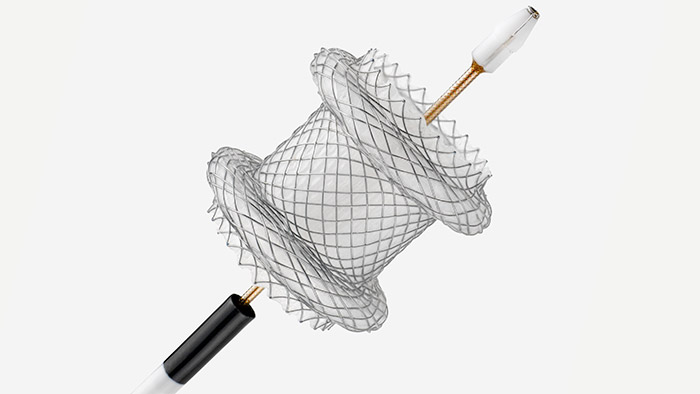
Enabling effcient and effective treatment procedures
At Boston Scientific, we work tirelessly to deliver innovative solutions that help you offer the best possible patient experience.