It is more important than ever for physicians to monitor and manage their heart failure patients.
Heart Failure diagnostics, battery longevity, and remote management systems from Boston Scientific combine to offer 360-degree clinical care and reduce hospitalizations and hospital visits.
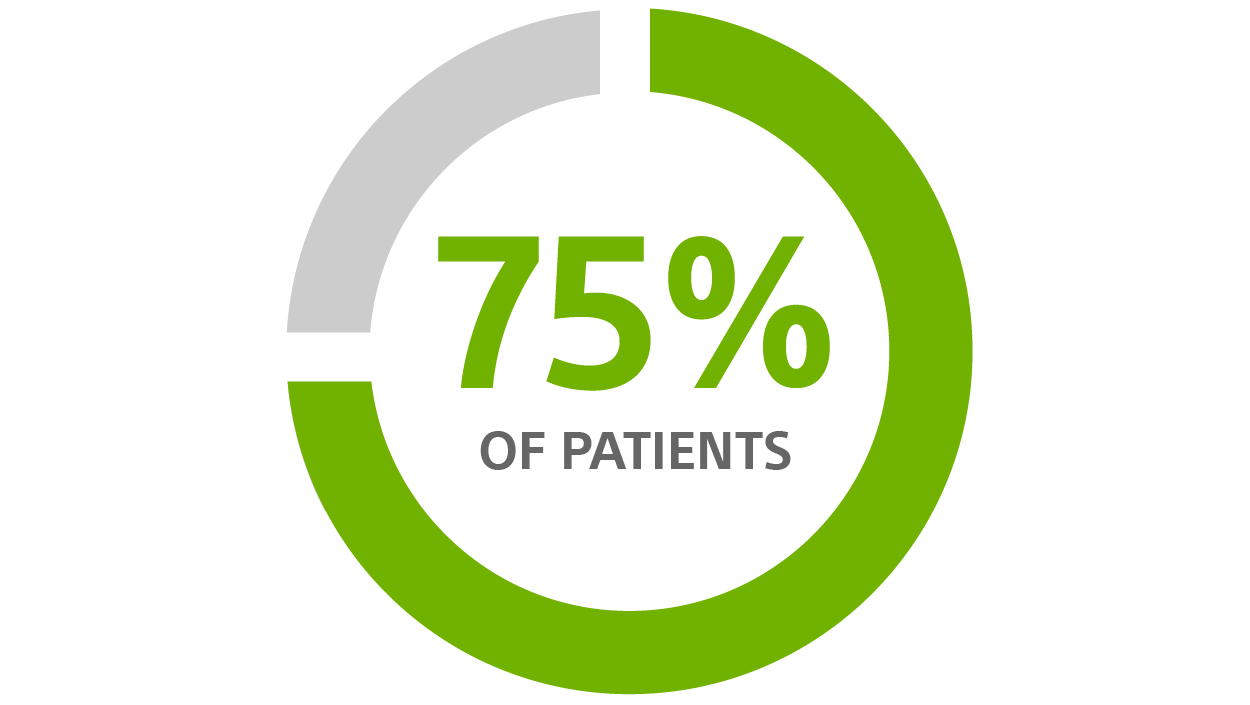
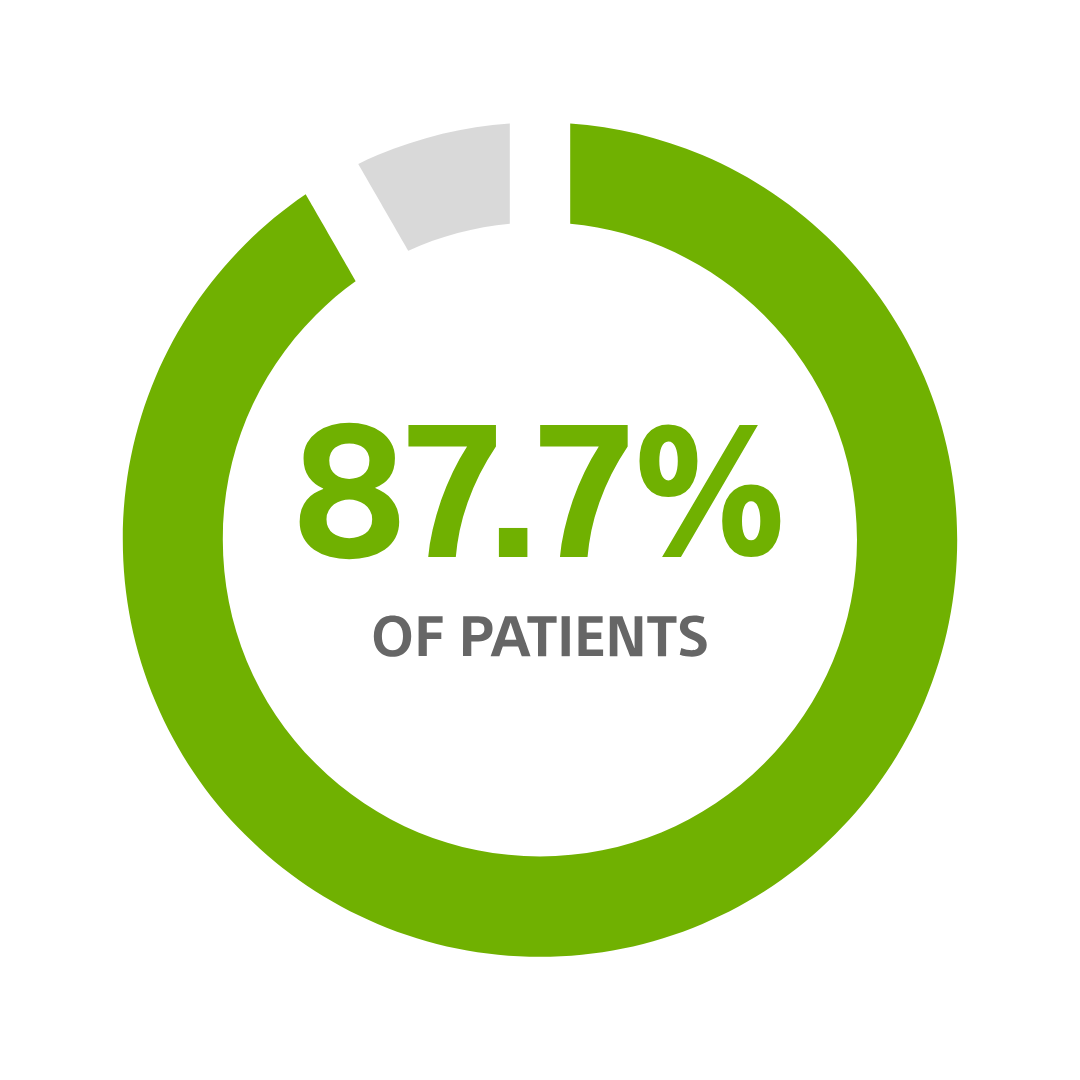
CRT-D avoidable change-outs for patients >70 years old, using a CRT-D with nine-year longevity¹
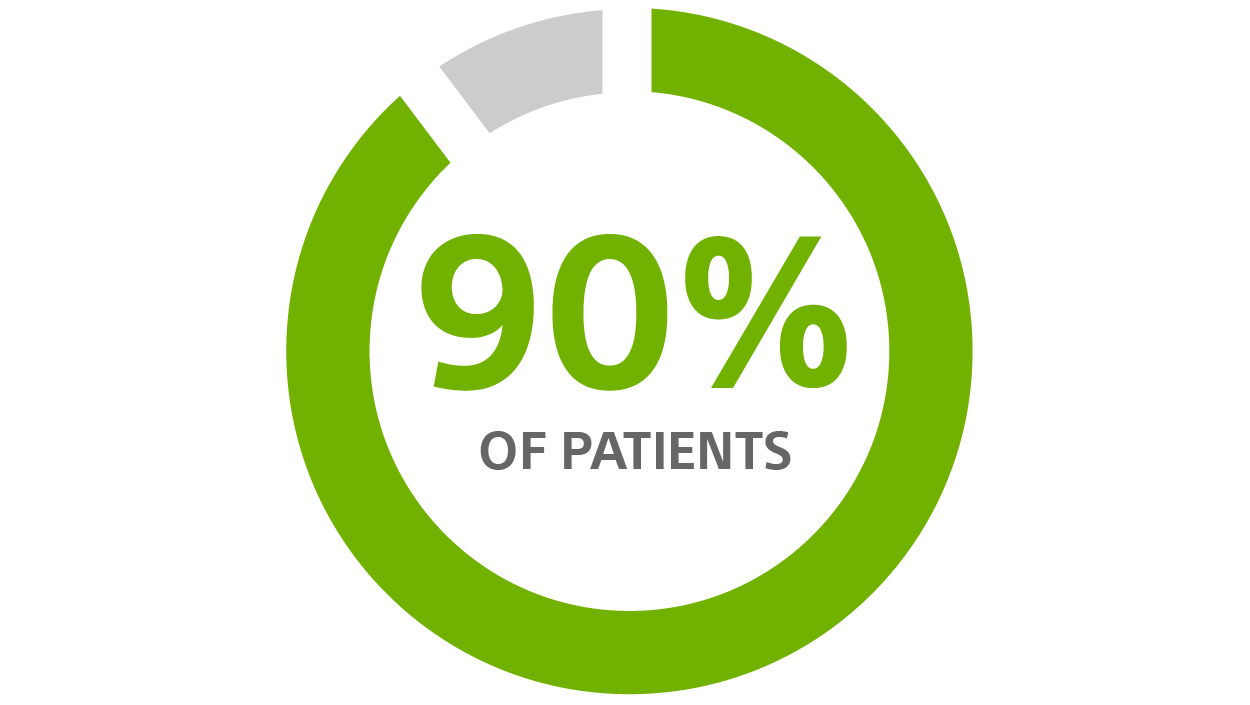
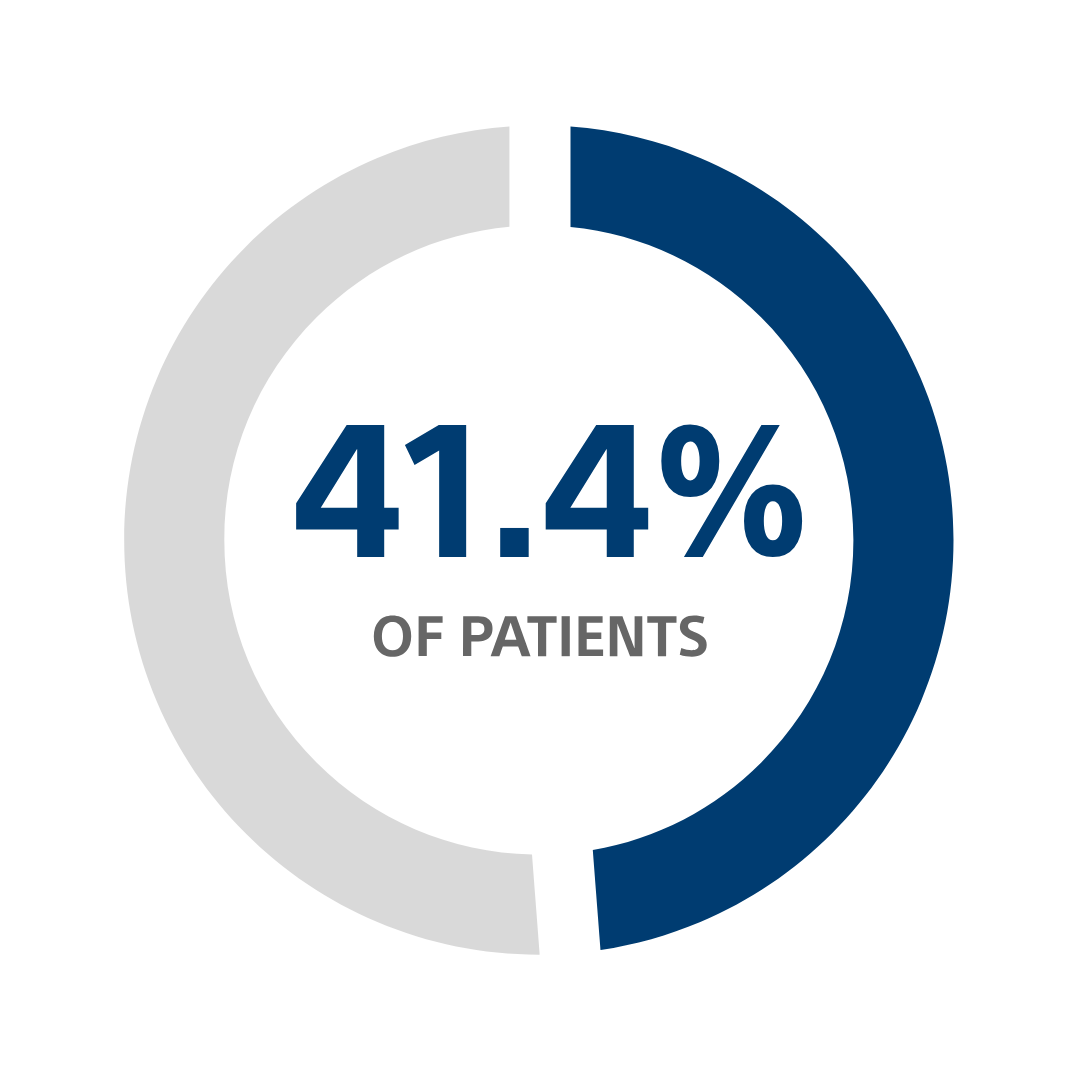
Patients desire a longer-lasting device²
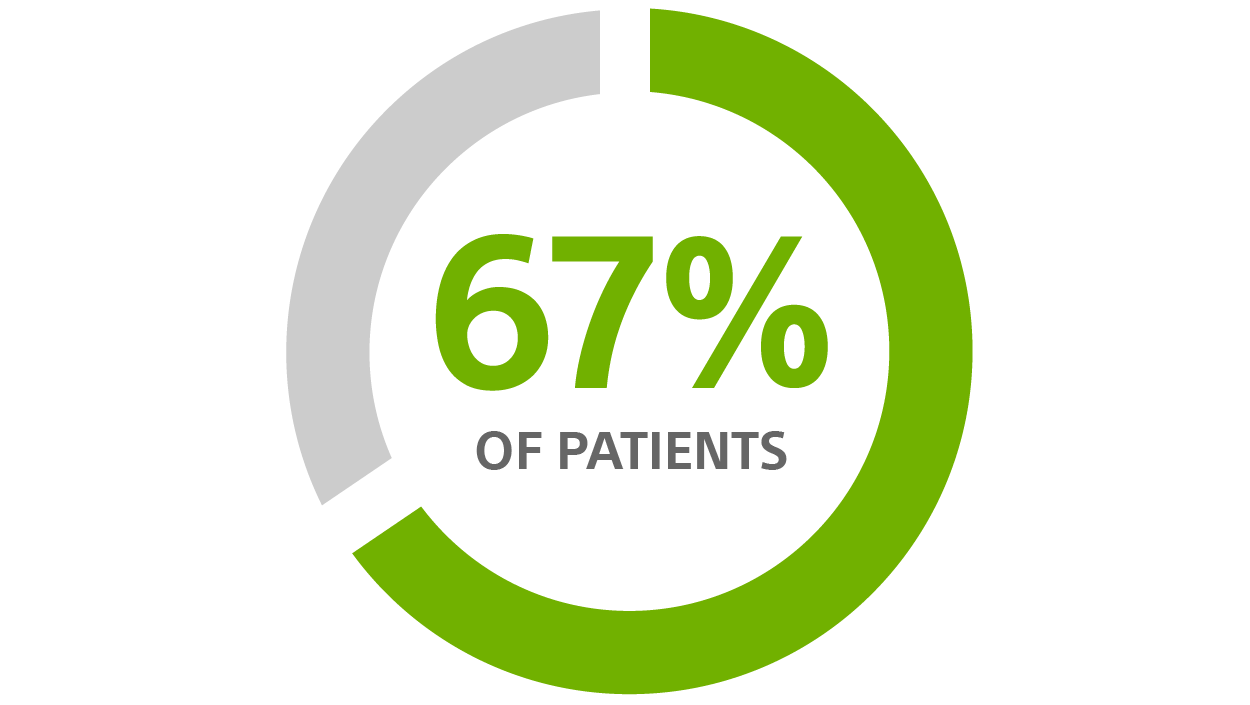
Reduction in hospitalizations associated with HeartLogic™³
Longevity
The patient's life is improved by longevity by requiring fewer replacements:
- Assuring that infection and complications are minimized
- Reducing the cost of therapy
- Enhancing patient therapy through battery optimization
- Improving patients' quality of life

EnduraLife™ Battery Technology was introduced by Boston Scientific in 2007 and has been validated by nine studies published since 20134⁴⁻¹³. Boston Scientific offers the longest-lasting devices on the market¹⁴⁻¹⁵.
Thanks to the ENDURALIFE technology, Boston Scientific ICD and CRT-D devices have demonstrated superior battery performance versus comparative devices, when estimated using standardized criteria⁵.

How we compare to our competitors
The 8-year product performance reports show that CRT-Ds with Enduralife Technology have greater longevity than comparable devices.¹⁷⁻¹⁸
BOSTON SCIENTIFIC
CRT-Ds still in service¹⁷
MEDTRONIC
Viva Quad XT CRT-D¹⁸
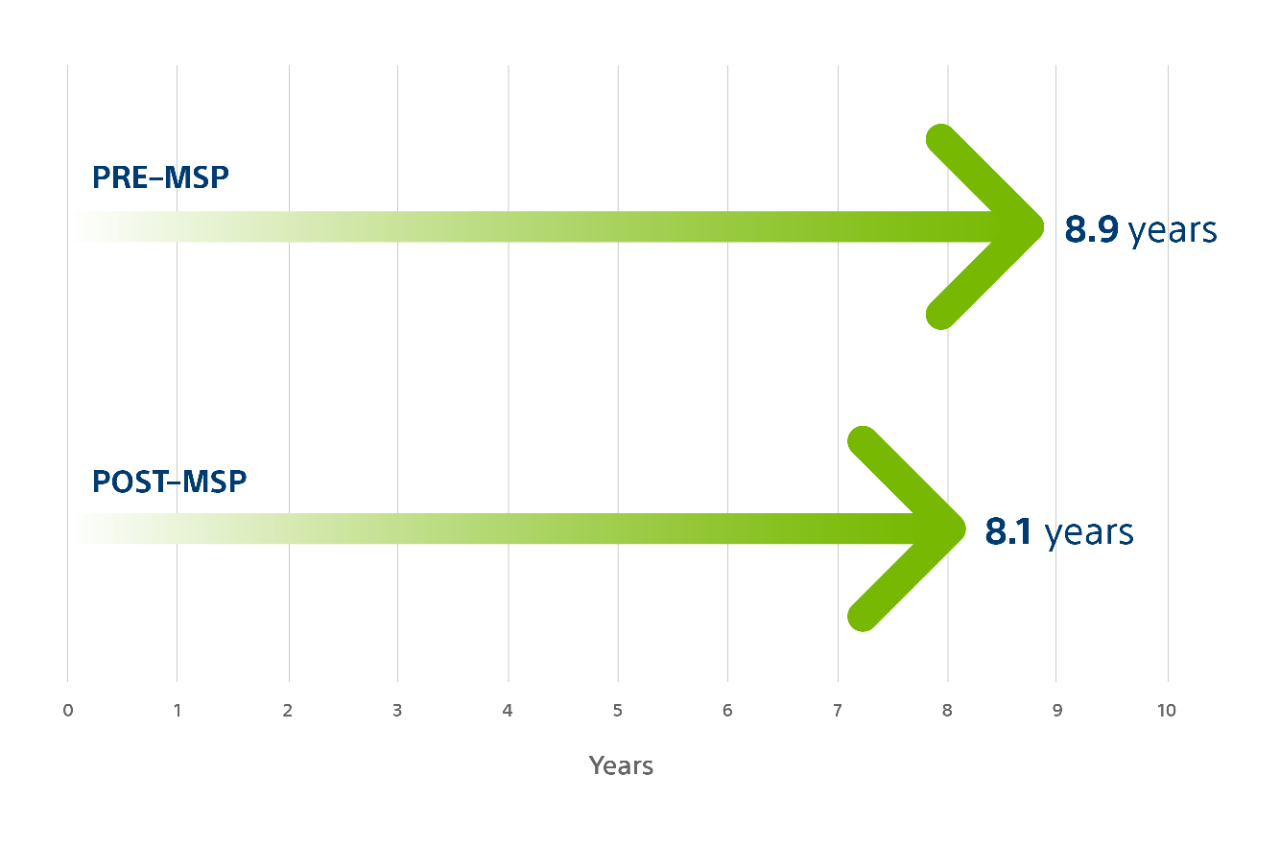
SMART Multi-Site Pacing (MSP)¹⁹
Based on the results of the SMART-MSP study conducted in the US with 584 patients, Boston Scientific Multi-Site Pacing is associated with improved clinical response in non-responders to CRT therapy, as well as minimal impact on battery life.
Multi-Site Pacing (MSP) has a minimal impact on battery longevity
Economic and Environmental Gains
Higher battery capacity and device longevity not only offer clinical benefits but also result in daily savings²⁰ ranging from 0.53€ for ICD VR to 1.48€ for CRTD with MSP on.


Higher device longevity is also associated with potential decrease of GHG emission and wastage thanks to the avoidance of substitution procedures (requiring additional patient journeys, hospitalization days and surgical procedures)²¹.

Discover more on EnduraLife Battery Technology
Remote Patient Management
Remote device monitoring is more relevant and useful than ever during the COVID-19 pandemic. It will help you:
- Identify clinical events and device anomalies in patients with cardiac implantable defibrillators who's in-person visit was canceled.
- Allow physicians to monitor disease progression remotely and to modify patients’ treatment appropriately (except for programming changes).
- When patients feel more empowered in the remote monitoring process, they will become more invested in improving their health. Thus, we should feel confident to use remote device monitoring for patients with cardiac implantable defibrillators during the COVID-19 pandemic²².

In the context of the Covid-19 pandemic, leveraging remote patient monitoring technology will help eliminate unnecessary patient contact, while reducing the burden on in-clinic follow-up.
- "Is recommended that HF patients are enrolled in a multidisciplinary HF management programme to reduce the risk of HF hospitalisation and mortality."²³
- "Remote device management is recommended to reduce number of in-office follow-up in patients."²³
2021 ESC Guidelines²⁴⁻²⁵ for the management of chronic Heart Failure and implantable devices. Recommendations have now the highest level of evidence: Class 1, level of evidence A
Discover Boston Scientific Patient Management solutions

Heart Failure Diagnostic
In the COVID-19 pandemic, and beyond, HeartLogic offers a personalised, remote heart failure diagnostic and monitoring solution. Using multiple, novel physiologic sensors with high sensitivity and low-alert burden, it’s validated to provide weeks of advance notice for detecting early signs of worsening heart failure.
HeartLogic is clinically validated to provide weeks of advance notice for detecting early signs of worseningheart failure²⁶
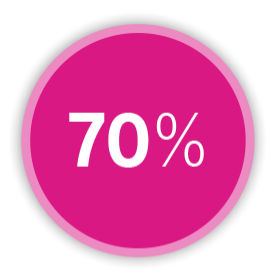
SENSITIVITY
in detecting heart failure events²⁶

DAYS ADVANCE NOTICE
of heart failure worsening²⁶

TOTAL ALERTS
per patient per year²⁶
Integration into clinical practice
In the MANAGE-HF Phase 1 Study²⁷, HeartLogic is associated with:
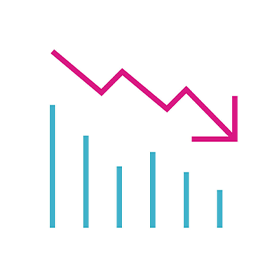
- A 67% reduction in HF hospitalisation rate
- A reduction of N-terminal pro b-type natriuretic peptide (NTproBNP) levels
MORE RAPID RECOVERY
of HeartLogic index
LOWER
NTproBNP Levels
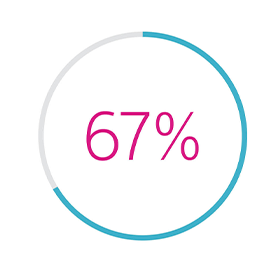
67% REDUCTION
in Hospitalisation Associated with HeartLogic
Discover how HeartLogic™ can help you in your daily practice


















