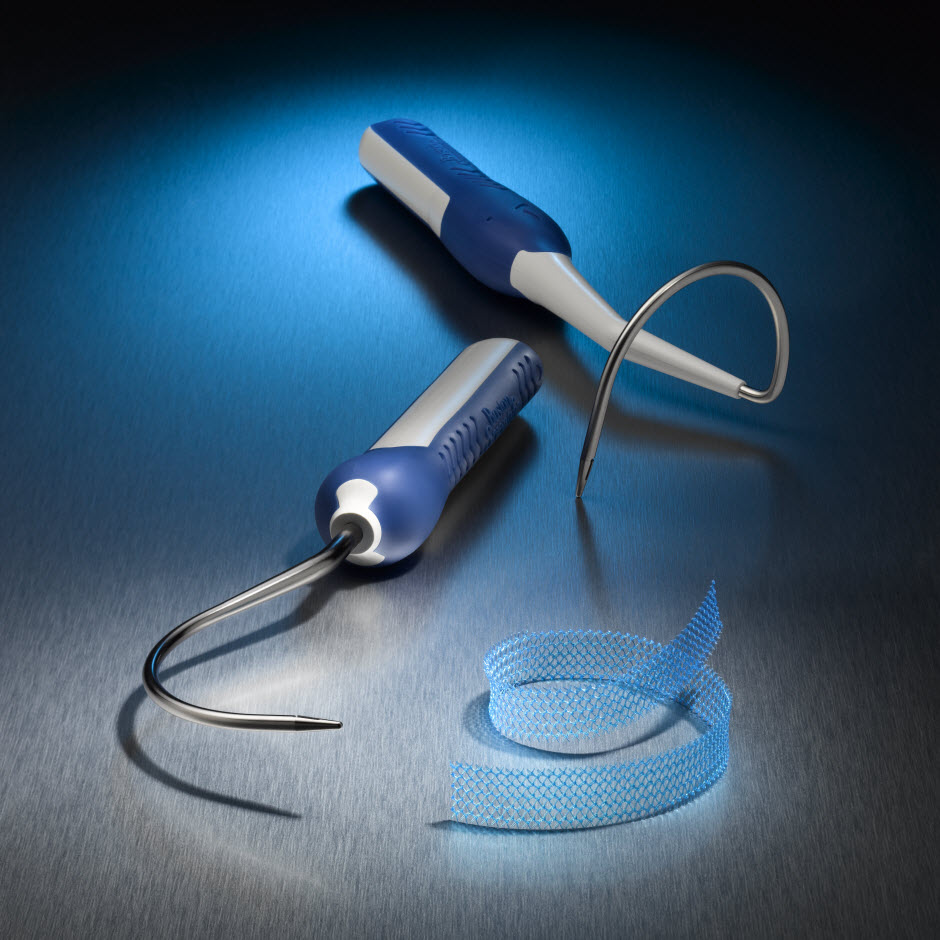
Obtryx™ II
Transobturator Mid-Urethral Sling System with PrecisionBlue™ Design
Transobturator sling with enhanced features that are designed to provide smooth sling placement, intra-operative adjustability designed for minimal tissue disruption, and increased physician visualization that aids in precise sling placement.
Key Resources
Explore
Get in touch with our team
Whether you’re looking to trial a product in our Ultra Sling Family or have general questions about the products, the ordering process or clinical data, our team is here to help.
Product Details
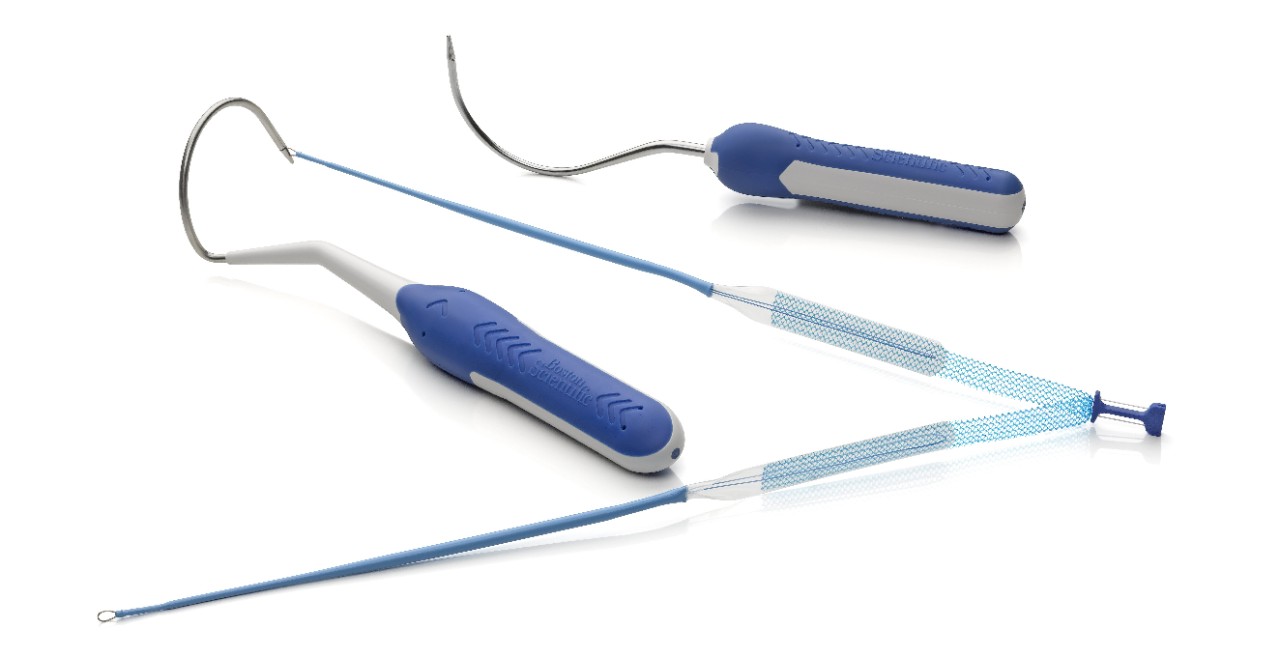
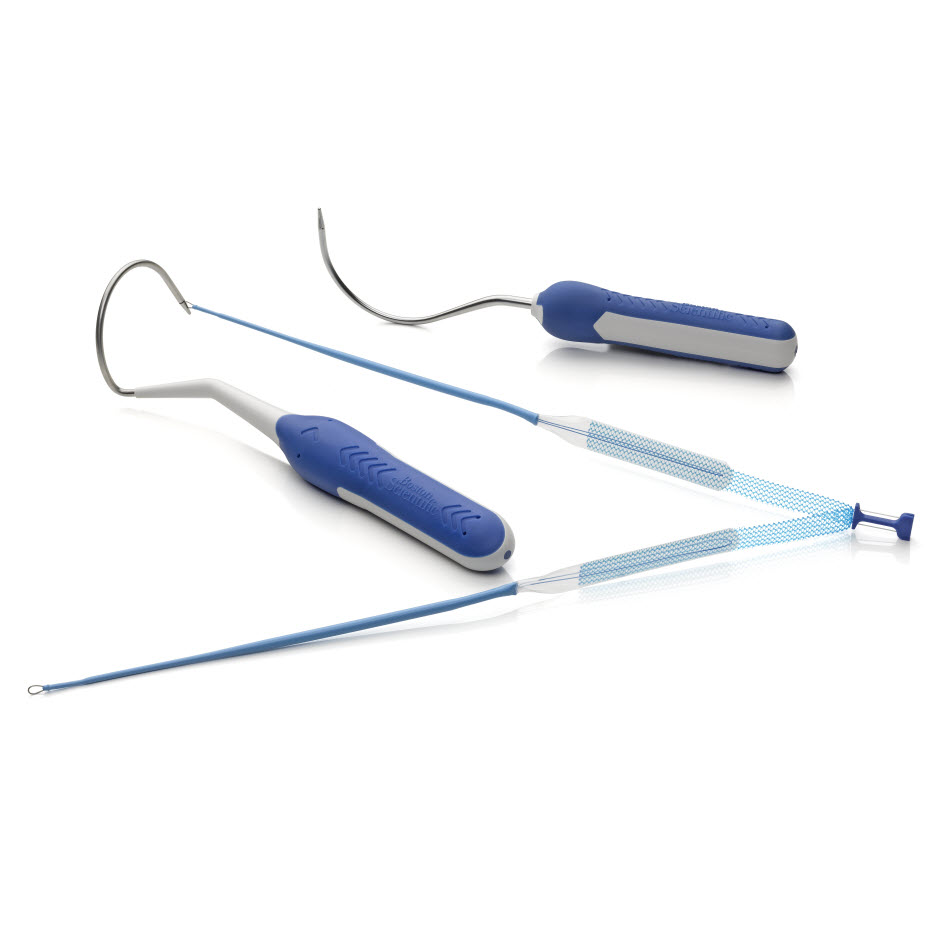
Needle Design
- Needle tip length is designed to facilitate transobturator device passage.
- Two needle configurations, halo and curved, allow physicians to choose the needle that meets their preference.
Association Loop
- Designed to facilitate needle engagement and removal.
Dilator Legs
- Designed to create a small delivery track due to thin leg size.
- Provides smooth delivery of the sling through the anatomy designed for minimal tissue disruption.
Sub-Urethral Segment
- No sleeve coverage under the sub-urethral segment to allow for visibility and to aid in precise placement.
Centering Tab
- Blue centering tab identifies the center of the mesh and provides for equal distribution of mesh on each side of the urethra.
- The centering tab provides the ability to visualize tensioning and centering during positioning and sleeve removal steps.
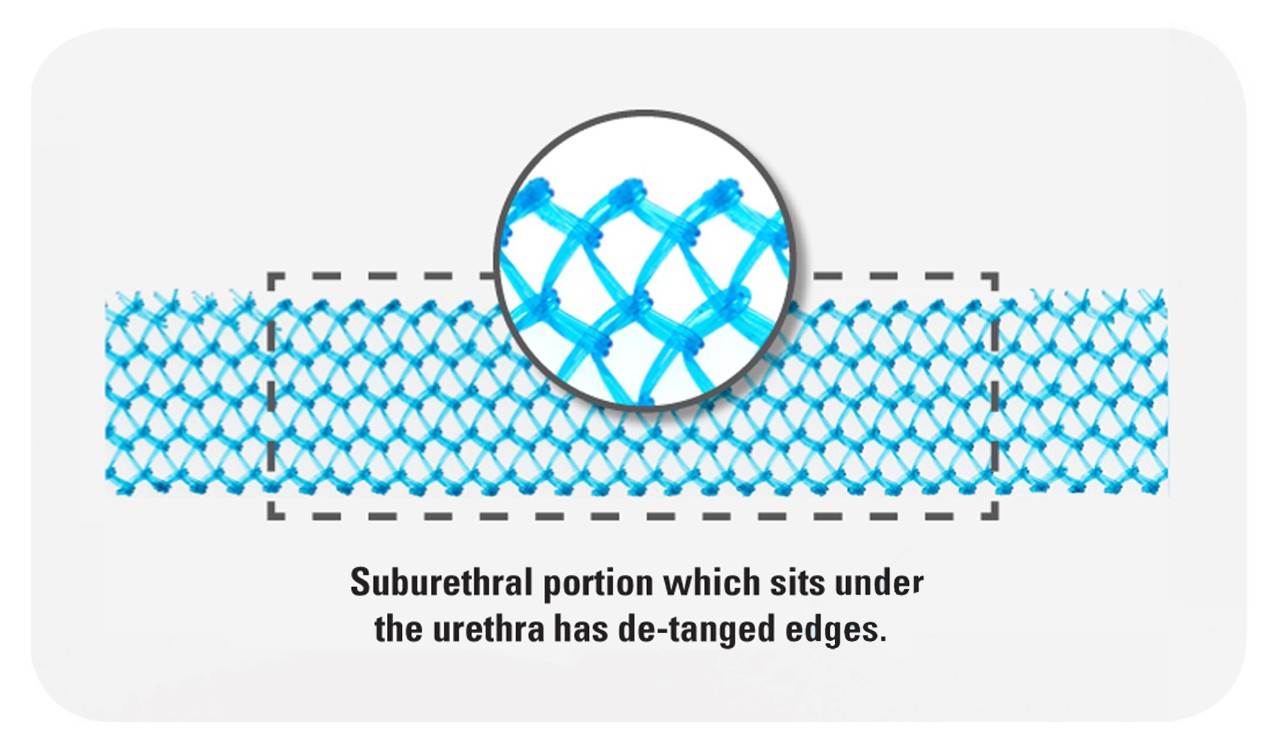
Blue Advantage™ Mesh
Detanged Polypropylene Material
Ordering Information
| Order Number | UPN | Description | Quantity |
|---|---|---|---|
| 850411 | M0068504110 | Obtryx II System | Single Unit |
| 850511 | M0068505110 | Obtryx II System | Single Unit |
Each System includes Two (2) Delivery Devices and One (1) Mesh Assembly