WATCHMAN FLX™ Pro
Left Atrial Appendage Closure Device
WATCHMAN FLX Pro is FDA approved for use in nonvalvular atrial fibrillation patients who are eligible for anticoagulation therapy. Built on the most studied and implanted LAAC device in the world, WATCHMAN FLX Pro is designed to enhance the healing process and optimize the therapy for more patients.
Key Resources
WATCHMAN FLX™ Pro Device
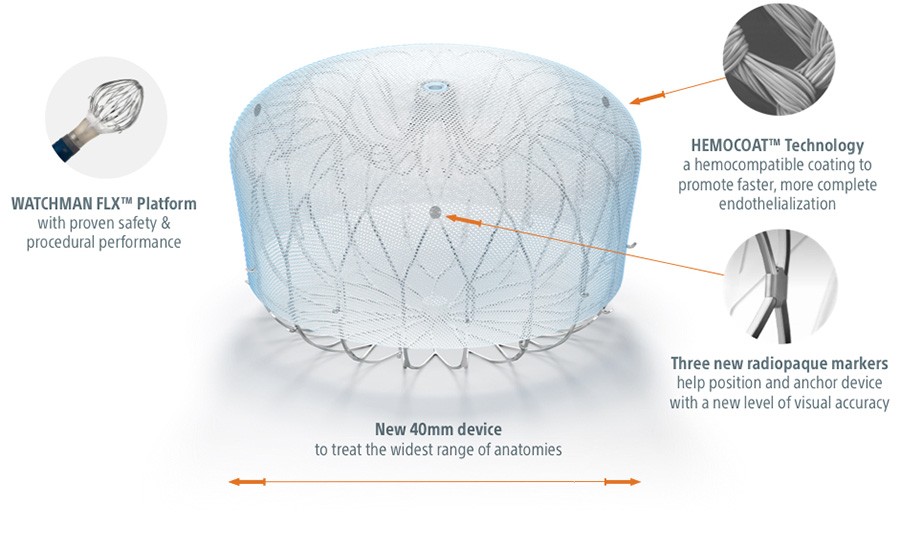
Coated for Controlled Healing
HEMOCOAT™ Technology is a durable, thromboresistant coating that results in less inflammation and leads to faster, more complete endothelialization.1
Next Level Visibility
Three new radiopaque markers help position and anchor the device with a new level of visual accuracy.
Close with Confidence
A new 40 mm size to treat larger appendages and widest range of anatomies.
