Identifying BT Candidates
Who is BT for?
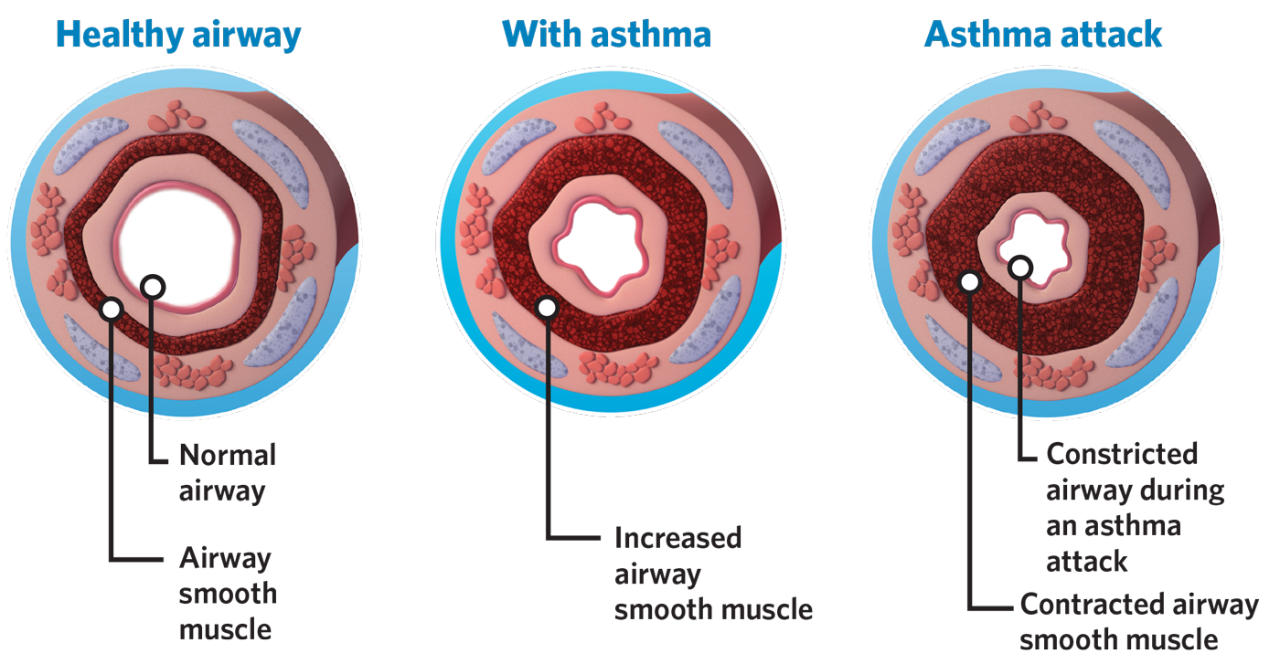
BT is for patients with severe asthma ≥ 18 years old who are on inhaled corticosteroids and long-acting beta2 agonists but still experience asthma symptoms and/or risk of future exacerbations.
These can include patients with any one of the following:
- Asthma exacerbations requiring oral corticosteroids
- ER, urgent care, or unscheduled office visits in the past 12 months
- Use of rescue inhaler > 2x per week
- Physical or activity limitations due to asthma
BT has 5 years of effectiveness and safety data
Learn more about the 5 year clinical safety data that demonstrate benefits of BT are maintained long term.
Click on the individual trial name, below, for additional information on each clinical study.
 |
 |
 |
| AIR2 5 Year Extension Study |
AIR2 Trial | RISA Trial |
Treatment With BT
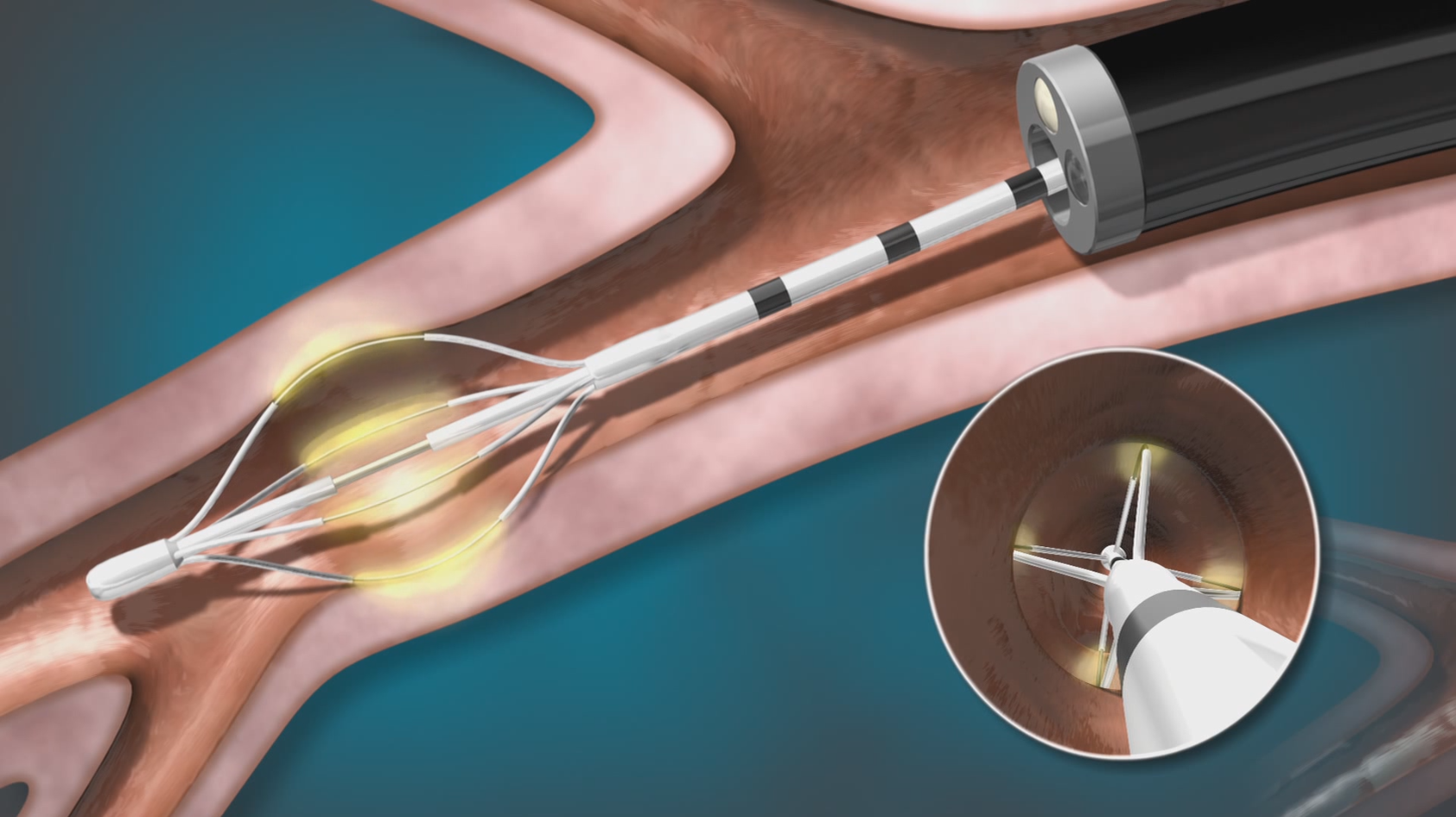
Performing BT
BT is delivered by the Alair™ System in 3 short sessions, using a device called the Alair™ Catheter. No incisions or full anesthesia necessary. Sessions are typically scheduled 3 weeks apart. Each session is routinely performed under moderate sedation and typically takes less than an hour to complete.