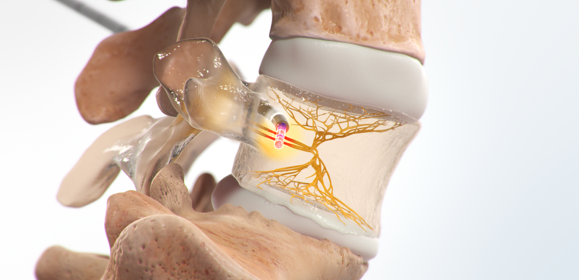
Advanced Interventions. Advancing Pain Management.
See What’s New from Boston Scientific
Key Events
Boston Scientific provides an essential selection of advanced therapies to treat chronic pain. In addition, our tailored business solutions help you and your staff overcome common challenges in practice management.
Clinical and Scientific Data Presentations
Pain Management
Deep Brain Stimulation
*The noted individual(s) serves as a consultant to Boston Scientific and is compensated for services provided under a consulting agreement.
U.S. Federal Government Employees – U.S. Federal Government Employees may be required to obtain approval from their agency’s or institution’s ethics officer or ethics committee or from a supervisor to attend this program. For more details, please contact your ethics officer or supervisor.
Vermont-Licensed HCPs – Vermont law prohibits Boston Scientific from providing any food, meals or refreshments at no charge to health care professionals licensed by and regularly practicing in Vermont. Accordingly, health care professionals licensed by and regularly practicing in Vermont are requested not to partake in any of the food, meals or refreshments offered at this event.
All U.S. Physicians – The U.S. Physician Payment Sunshine Act requires all pharmaceutical, biologics and medical device companies to disclose annually to the U.S. government payments and transfers of value provided to U.S. physicians and teaching hospitals. This includes the value of meals and refreshments provided to U.S. physicians in connection with attending Boston Scientific educational programs.
† Superion™ Indirect Decompression System
The WaveWriter Alpha™ SCS System provides safe access to full-body MRI scans when used with specific components and the patient is exposed to the MRI environment under the defined conditions in the ImageReady™ MRI Full Body Guidelines for WaveWriter Alpha and WaveWriter Alpha Prime Spinal Cord Stimulator System.
FAST MOA computational modeling by Gilbert JE, Titus N, Zhang T, Esteller R, Grill WM. Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation: Modeling and Measurement. eNeuro. 2022 Oct 5;9(5):ENEURO.0058-22.2022.
Sub-perception stimulation has been demonstrated to be safe and effective in patients who have been treated successfully with conventional, paresthesia-inducing stimulation for at least six months. Full stimulation parameter ranges and options for both paresthesia based and sub-perception therapy are available for clinician’s use throughout the patient’s experience and treatment with SCS.
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
Indications for Use: The Boston Scientific Spinal Cord Stimulator Systems are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs including unilateral or bilateral pain associated with the following: failed back surgery syndrome, Complex Regional Pain Syndrome (CRPS) Types I and II, Diabetic Peripheral Neuropathy of the lower extremities, intractable low back pain and leg pain. Associated conditions and etiologies may be: radicular pain syndrome, radiculopathies resulting in pain secondary to failed back syndrome or herniated disc, epidural fibrosis, degenerative disc disease (herniated disc pain refractory to conservative and surgical interventions), arachnoiditis, multiple back surgeries. Contraindications, warnings, precautions, side effects. The SCS Systems are contraindicated for patients who: are unable to operate the SCS System, have failed trial stimulation by failing to receive effective pain relief, are poor surgical candidates, or are pregnant. Refer to the Instructions for Use provided with the SCS System or Pain.com for potential adverse effects, warnings, and precautions prior to using this product.
Warning: Stimulation modes. Only paresthesia-based stimulation mode has been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Indications for Use: The Superion™ Indirect Decompression System (IDS) is indicated to treat skeletally mature patients suffering from pain, numbness, and/or cramping in the legs (neurogenic intermittent claudication) secondary to a diagnosis of moderate degenerative lumbar spinal stenosis, with or without Grade 1 spondylolisthesis, having radiographic evidence of thickened ligamentum flavum, narrowed lateral recess, and/or central canal or foraminal narrowing. The Superion™ Interspinous Spacer is indicated for those patients with impaired physical function who experience relief in flexion from symptoms of leg/buttock/groin pain, with or without back pain, who have undergone at least 6 months of non-operative treatment. The Superion Interspinous Spacer may be implanted at one or two adjacent lumbar levels in patients in whom treatment is indicated at no more than two levels, from L1 to L5. Contraindications, warnings, precautions, side effects. The Superion Indirect Decompression System (IDS) is contraindicated for patients who: have spinal anatomy that prevent implantation of the device or cause the device to be unstable in situ (i.e., degenerative spondylolisthesis greater than grade 1), Cauda equina syndrome, or prior decompression or fusion at the index level. Refer to the Instructions for Use provided on www.vertiflex.com for additional Indications for Use, contraindications information and potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Indications for Use: The Boston Scientific Radiofrequency Generators, associated Radiofrequency Lesion Probes and RF Cannula are indicated for use in procedures to create radiofrequency lesions for the treatment of pain or for lesioning only peripheral nerve tissue for functional neurosurgical procedures. The Boston Scientific RF Injection Electrodes are used for percutaneous nerve blocks with local anesthetic solution or for radiofrequency lesioning of peripheral nerve tissue only. The Boston Scientific LCED and Stereotactic TCD Electrodes are indicated for use in radiofrequency (RF) heat lesioning of nervous tissue including the Central Nervous System. Warnings: The Boston Scientific RF devices may cause interference with active devices such as neurostimulators, cardiac pacemakers, and defibrillators. Interference may affect the action of these active devices or may damage them. For appropriate guidance, consult the instructions for use for these active devices. Refer to the Instructions for Use provided with Boston Scientific generators, electrodes and cannulas for potential adverse effects, warnings and precautions prior to using these products.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Indications for Use: The Intracept Procedure Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change). Contraindications - Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in: Patients with severe cardiac or pulmonary compromise, patients with active implantable pulse generators (e.g. pacemakers, defibrillators), patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal), patients with active systemic infection or local infection in the area to be treated, patients who are pregnant, and/or skeletally immature patients (generally ≤ 18 years of age). Refer to the Instructions for Use provided with the Intracept Procedure or www.relievant.com/intracept/ for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Indication for Use: The Boston Scientific Vercise™ PC, Vercise Gevia™, Vercise Genus™ Deep Brain Stimulation Systems are indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Bilateral stimulation of the internal globus pallidus (GPi) as an adjunctive therapy in reducing some of the symptoms of advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Unilateral thalamic stimulation of the ventral intermediate nucleus (VIM) is indicated for the suppression of tremor in the upper extremity. The system is intended for use in patients who are diagnosed with essential tremor or parkinsonian tremor not adequately controlled by medications and where the tremor constitutes a significant functional disability.
The Boston Scientific Vercise Deep Brain Stimulation System is indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Contraindications, warnings, precautions, side effects: The Boston Scientific Deep Brain Stimulation (DBS) Systems or any of its components, are contraindicated for: Diathermy as either a treatment for a medical condition or as part of a surgical procedure, Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) as the safety of these therapies in patients implanted with the Boston Scientific DBS System has not been established, patients who are unable to operate the system, patients who are poor surgical candidates or who experience unsuccessful test stimulation. Patients implanted with Boston Scientific DBS System without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Patients implanted with Vercise Gevia or Vercise Genus or Vercise Genus Mixed System with M8 Adapter or Vercise DBS Lead-Only System (before Stimulator is implanted) with ImageReady MRI Technology are Full Body MR Conditional only when exposed to the MRI environment under the specific conditions defined in ImageReady MRI Guidelines for Boston Scientific DBS Systems. Assess patients for the risks of depression and suicide. This assessment should consider both the risk of depression and suicide as well as the potential clinical benefits of DBS therapy. Monitor patients for new or worsening symptoms of depression, suicidal thoughts or behaviors, or changes in mood or impulse control and manage appropriately. Refer to the Instructions for Use provided with the Boston Scientific DBS Systems or BostonScientific.com for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.