Detachable Coils Portolio
Solutions for FRAMING, FILLING and
FINISHING in Peripheral Embolization
The IDC™ Interlocking Occlusion Coil family offers bare platinum detachable micro-coils with softer design than standard embolization coils, for tight packing and nesting into the vessel and into the coil frame for specific embolization needs.
Key Resources
Explore
Product details
Two rigidity levels are available:
- IDC Regular to complete Interlock packing
Designed for smooth and tight packing of the coil in combination with high thrombogenicity and filling capability of Interlock
- IDC Soft for smooth and elegant finishing
Designed for smooth and tight packing during last coil delivery to prevent catheter kickback and last compaction in the target vessel as well as for extra-small vessels.
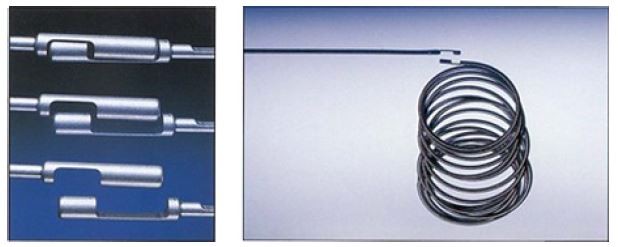
Each coil is mechanically attached to a coil delivery wire by “interlocking arms”. This simple and elegant design allows the coil to be advanced and retracted before final placement in the vessel, thus aiding in more controlled delivery.”
Soft Coil Design
- Thin wire and soft primary coil wind.
- Designed for smooth, tight nesting of the coil, and reduced risk of catheter kickback, for delicate and accurate finishing during vessel embolization
Precise Placement
- Interlocking arms offer ability to retract and reposition before detaching, for precise placement
Familiar Technology with Clinical Versatility
- Same detachment mechanism and microcatheter compatibility as Interlock-18
- Range of sizes (from extra-small 2mm x 2cm to extra-long) to facilitate use in extra-small peripheral vessels, delicate finishing needs, and for Hght packing in large aneurysms
Ordering information
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M0033612020 | IDC-18 Soft | 2 | 20 | 2D |
| M0013612040 | IDC-18 Soft | 2 | 40 | 2D |
| M0013613060 | IDC-18 Soft | 3 | 60 | 2D |
| M0013613100 | IDC-18 Soft | 3 | 100 | 2D |
| M0013614040 | IDC-18 Soft | 4 | 40 | 2D |
| M0013614080 | IDC-18 Soft | 4 | 80 | 2D |
| M0013614120 | IDC-18 Soft | 4 | 120 | 2D |
| M0013615080 | IDC-18 Soft | 5 | 80 | 2D |
| M0013615120 | IDC-18 Soft | 5 | 120 | 2D |
Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for the use only in countries with applicable health authority product registrations. Not for use or distribution in France.