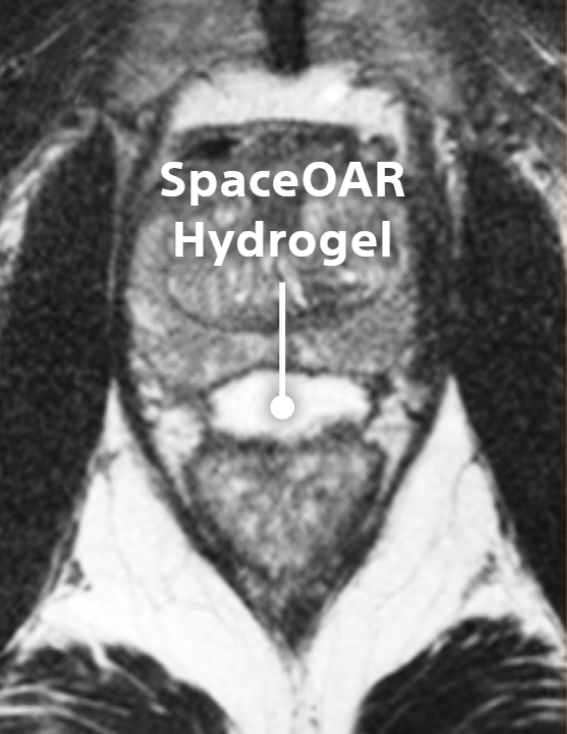
SpaceOAR™ Hydrogel
When treating prostate cancer patients with radiation therapy, the goal is to destroy the cancer cells while avoiding damage to surrounding healthy tissue.
SpaceOAR provides an 1.3cm space between the prostate and rectum for prostate cancer radiation patients to help reduce radiation complications.
Want to learn more about how to advance radiation oncology?
Minimising side effects of radiation therapy
![]() Better patient bowel quality of life in late follow-up exceeding the threshold for a minimal clinically important difference (mean difference = 5.4)
Better patient bowel quality of life in late follow-up exceeding the threshold for a minimal clinically important difference (mean difference = 5.4)
![]() 66% less v70 rectal irradiation
66% less v70 rectal irradiation
![]() 70% reduction in the risk of rectal toxicity (grade ≥ 1) in late follow-up
70% reduction in the risk of rectal toxicity (grade ≥ 1) in late follow-up
![]() 77% reduction in the risk of rectal toxicity (grade ≥ 2) in late follow-up
77% reduction in the risk of rectal toxicity (grade ≥ 2) in late follow-up
In a pooled analysis of 1,011 patients receiving radiotherapy from 7 clinical studies, SpaceOAR Hydrogel when compared to control demonstrated lower rectal toxicity and improved bowel quality of life.
Clinical trials in the U.S2,3,4 and Europe5,6 have demonstrated that SpaceOAR Hydrogel is safe and that the space created with hydrogel spacers significantly reduces the radiation delivered to the rectum.
The randomised SpaceOAR Hydrogel U.S. Clinical Trial found that patients who received SpaceOAR Hydrogel reported significantly less rectal pain during radiotherapy2 and had significantly fewer severe long-term rectal complications.3,4
High-dose Ultrahypofractionated Radition Therapy
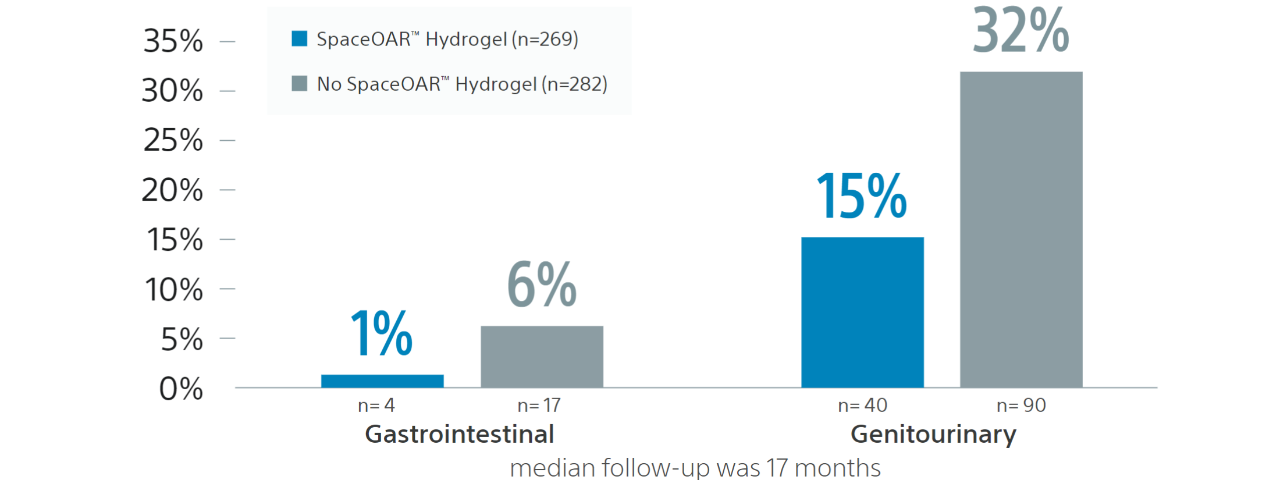
In a retrospective analysis entitled Early Tolerance and Tumor Control Outcomes with High-Dose Ultrahypofractionated Radiation Therapy for Prostate Cancer, a total of 551 prostate cancer patients received dose-escalated SBRT (40 Gy over 5 fractions). 269 of the 551 patients received a hydrogel spacer.
The incidence of grade 2+ late rectal toxicity in the hydrogel and nonhydrogel spacer cohorts was 1% and 6%, respectively (p=0.01). The incidence of grade 2+ late GU toxicity in the hydrogel and nonhydrogel spacer cohorts was 15% and 32%.
“The use of a hydrogel rectal spacer was significantly associated with reduced late GI toxicity and lower odds of developing late GU toxicity”7
SBRT and patient pathways in a COVID-19 environment
In association with the ESTRO 2020 virtual congress, watch the radiation oncology experts Prof. Fillipo Alongi and Prof. Michael Zelefsky explaining SBRT with Hydrogel Spacing during COVID-19.
SpaceOAR Hydrogel received positive NICE guidance

The use of a hydrogel spacer received positive NICE guidance (IPG590) for the reduction of rectal toxicity during radiotherapy for prostate cancer based on the results of the SpaceOAR Hydrogel US Pivotal Clinical Trial.