Boston Scientific accounts are for healthcare professionals only.




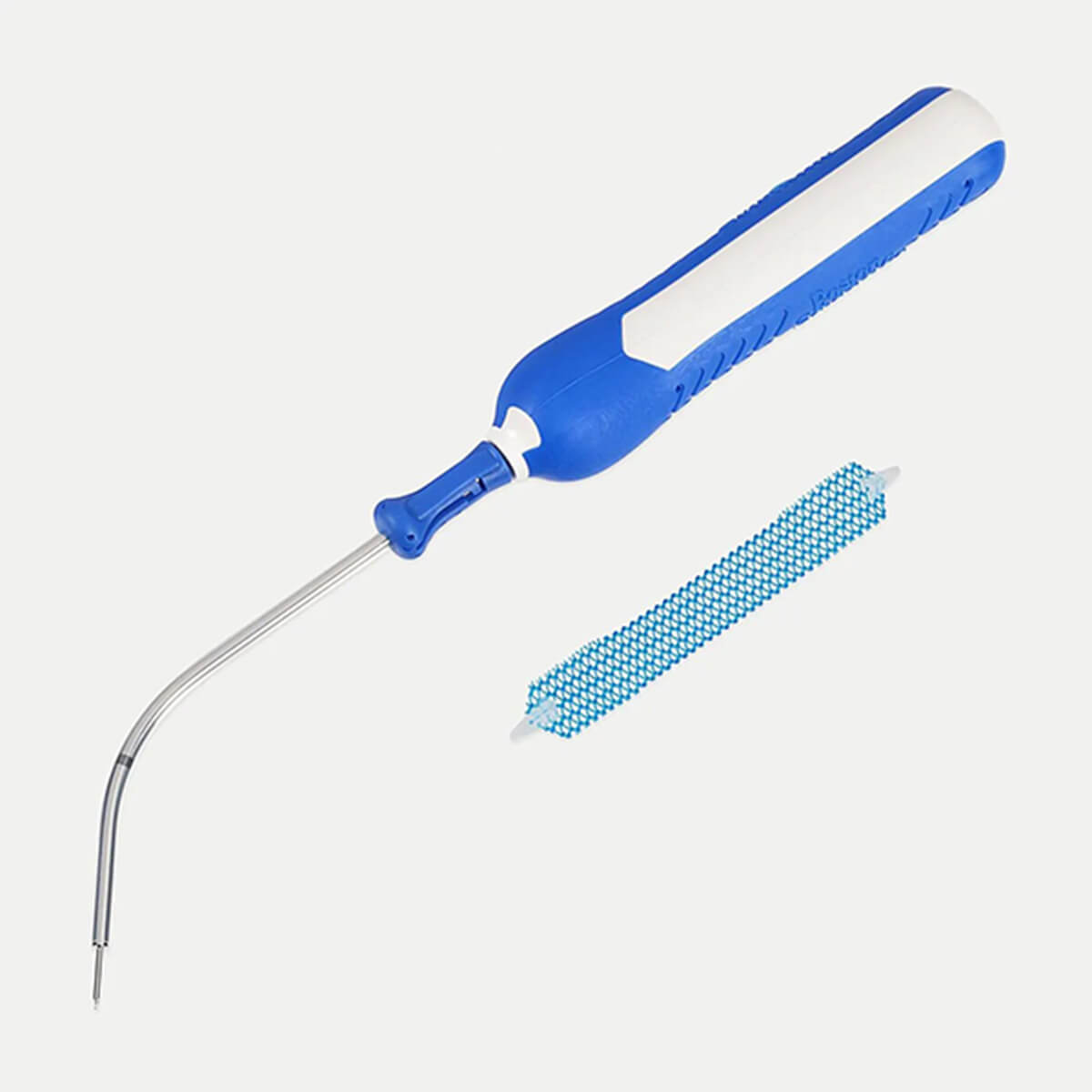
Solyx™ Single Incision Sling System
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Training
- Resources
- Ordering information
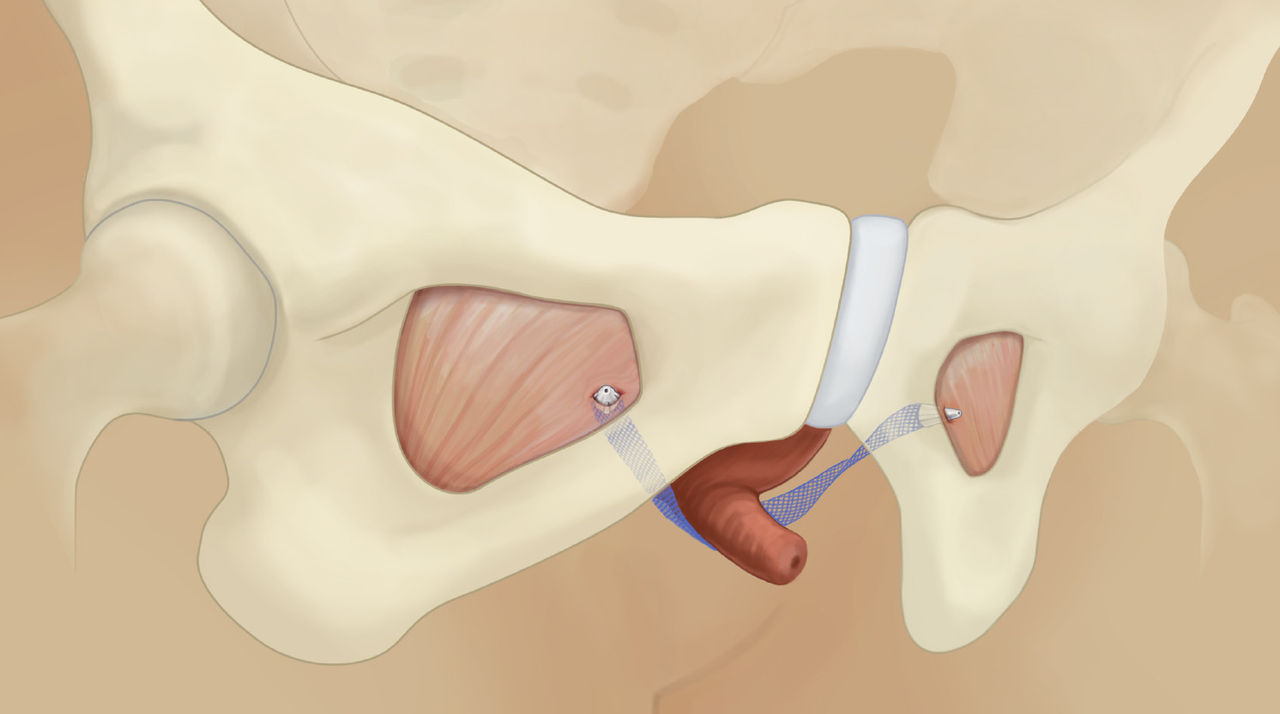
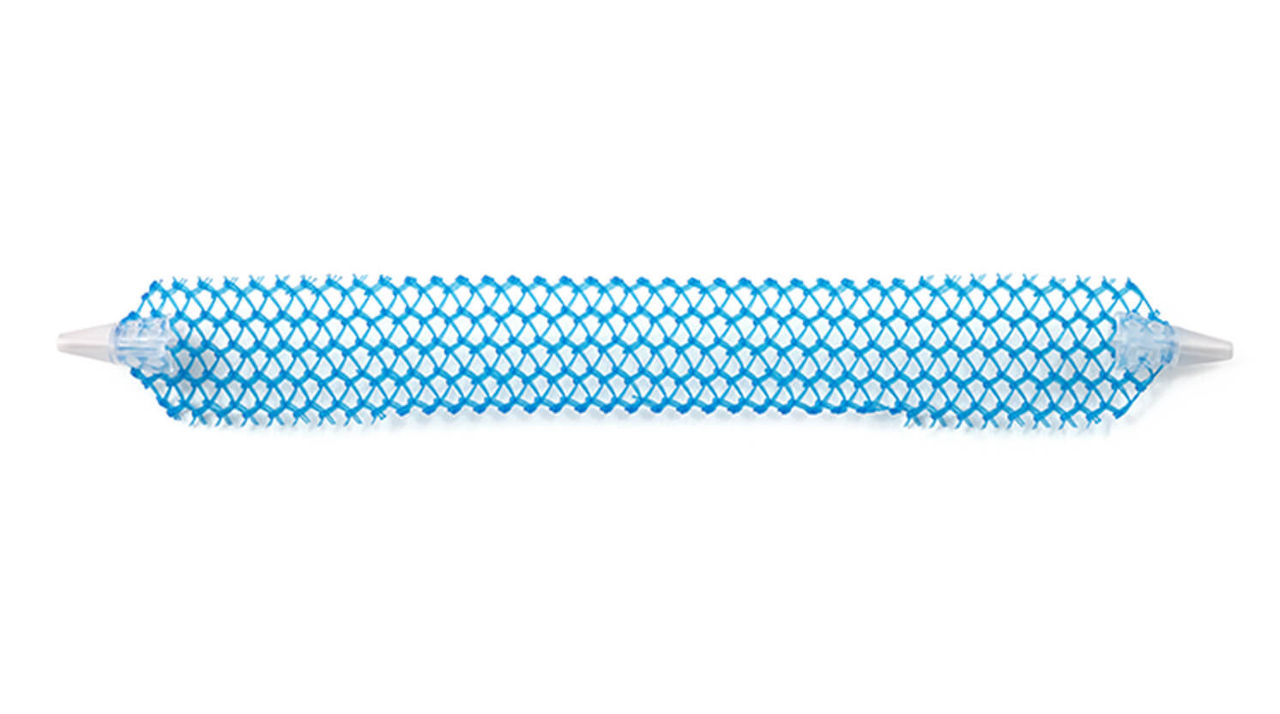
The single incision sling system designed with mesh characteristics, fixation, and adjustability in mind
Solyx is designed for secure fixation while allowing for intra-operative tensioning and adjustments. Advantage mesh is documented in more than 100 publications to date and has been used in over 1 million Advantage products.
Take a closer look at the key features of the Solyx sling
Why choose Solyx™?
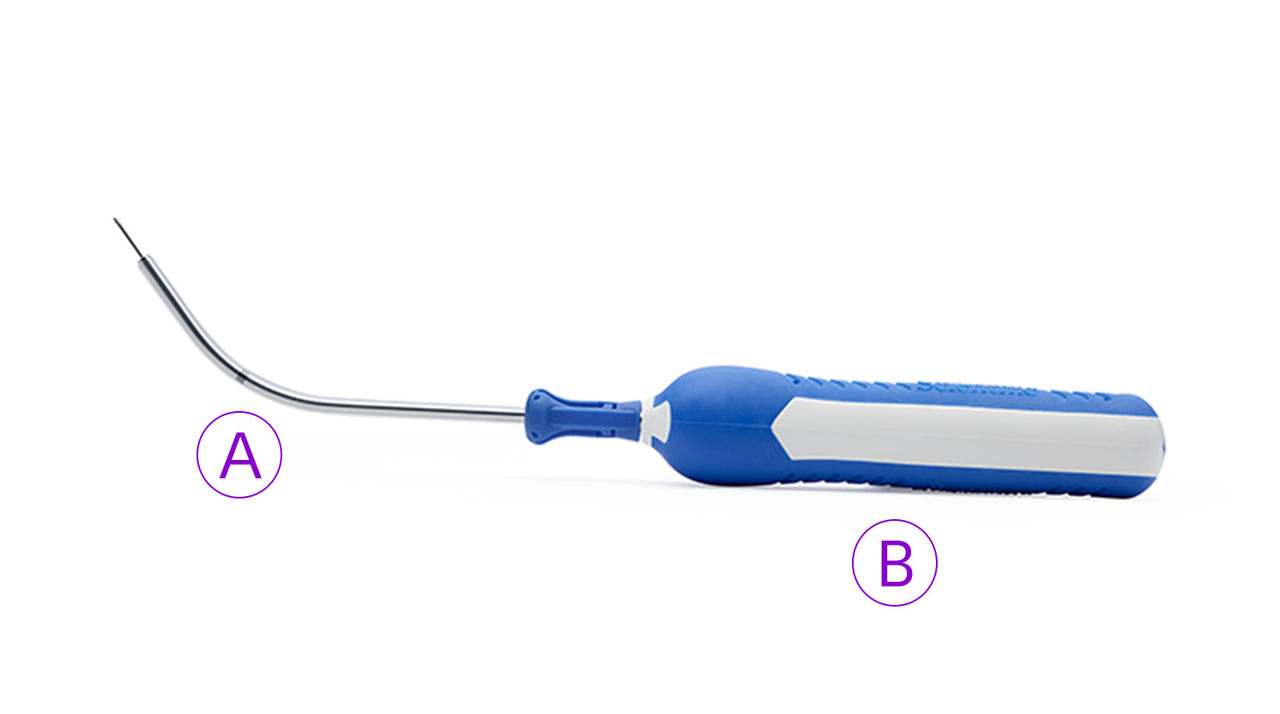
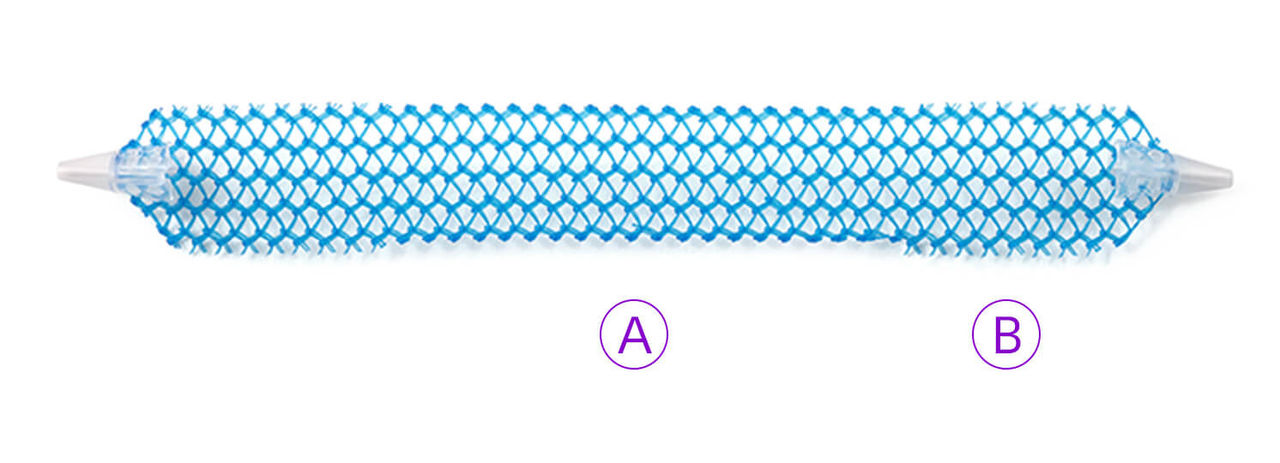
What’s included
Studies in progress
Sponsor: Wake Forest University Health Sciences
Brief Summary: SASS (Single-incision Versus Retropubic Mid-Urethral Sling (Solyx) for SUI During Minimally Invasive Sacrocolpopexy) is a multicenter, prospective, randomized, single-blind non-inferiority trial. SASS aims to compare the efficacy of a single-incision (SIS) versus a retropubic mid-urethral sling (RP) placed at the time of minimally invasive sacrocolpopexy in women with pelvic organ prolapse and objectively confirmed stress urinary incontinence (SUI).
Sponsor: NICHD Pelvic Floor Disorders Network
Brief Summary: This is a multicentered, double-blind, randomized controlled, surgical trial of 358 women with inadequate symptom relief of stress urinary incontinence (SUI) or stress predominant mixed urinary incontinence (MUI) after conservative care. The Primary Aim is to determine the comparative effectiveness (as defined by “much” or “very much” better on PGI-I) of transurethral bulking agent (TBA) [for 1 or 2 injections in 12 months] vs. single-incision sling (SIS) 12 months after treatment intervention in women with predominant stress urinary incontinence (SUI).
Featured clinical publications
White AB, Kahn BS, Gonzalez RR, Rosamilia A, Anger JT, Eilber KS, Schaffer JI. Prospective study of a single-incision sling versus a transobturator sling in women with stress urinary incontinence: 3-year results. Am J Obstet Gynecol. 2020;223:545.e1–545.e11.
Gonzalez RR, Rosamilia A, Eilber K, Kahn BS, White AB, Anger JT. Three-year patient-reported outcomes of single-incision versus transobturator slings for female stress urinary incontinence are equivalent. Int Urogynecol J. 2023;34:2265–2274.
White AB, Anger JT, Eilber K, Kahn BS, Gonzalez RR, Rosamilia A. Female sexual function following sling surgery: a prospective parallel cohort, multi-center study of the Solyx™ Single Incision Sling System versus the Obtryx™ II Sling System. J Urol. 2021;206:696–705.
Serels S, Douso M. Long term follow up of the Solyx Single Incision Sling in the treatment of female stress urinary incontinence (SUI). Open J Urol. 2014 Feb.
Lau HH, Enkhtaivan S, Su TH, Huang WC. The outcome of a sngle-incision sling versus trans-obturator sling in overweight and obese women with stress urinary incontinence at 3-year follow-up. J Clin Med. 2019;8:1099.
Technical specifications
| Mesh | |
| Mesh length | 9 cm |
| Mesh thickness | 0.66 mm |
| Pore size | 1182 μm |
| Fiber size (diameter) | 0.15 mm |
| Weight | 100 g/m2 |
Training for Solyx™
Online medical training and education courses
The EDUCARE online platform makes health care education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
Testimonials
If you treat patients with stress urinary incontinence, you know the journey can be long and life changing. Often, women wait years before they make their first appointment, but when they do seek treatment, their lives can be improved.
Explore the journey from initiating the conversation to seeing lives impacted by the Solyx™ Single Incision Sling in this short video series.
Episode 1: Beginning the conversation
When evaluating patients with stress urinary incontinence and assessing treatment options, Dr. Kevin Benson highlights the importance of patient history and personal goals. Hear why he often recommends the Solyx™ Single Incision Sling including his focus on patient outcomes and recovery time.
Episode 2: Evaluating for treatment
For AJ and Chandra, their stress urinary incontinence journey began after childbirth. Both dealt with the problem for years — wearing pads and avoiding activities they enjoyed — until they decided to seek treatment and get their lives back. Hear what drove them to take the next step.
Episode 3: Back to enjoying life
Six weeks after having the Solyx™ Single Incision Sling placed, Chandra says it was “like starting my life over,” and AJ recalls her “self-esteem was back up.” See how the procedure impacted their lives and allowed them to return to some of their favorite activities. Also, hear about other patient feedback on the recovery journey, as shared by Dr. Kevin Benson.
Product brochure
Patient resources
Ordering information
Order number | Description | Quantity |
M0068507000 | Solyx™ Single Incision Sling System | 1 Delivery Device and 1 Mesh Assembly |
M0068507010 | Solyx™ Blue Single Incision Sling System | 1 Delivery Device and 1 Mesh Assembly |
Please note that access to certain information on the following pages is intended for health care professionals.
For female Mid-Urethral Slings: CAUTION: Federal (US) law restricts this device to sale by or on the order of a physician trained in the use of surgical mesh for repair of stress urinary incontinence.
Refer to package insert provided with this product for complete Indications for use, contraindications, warnings, precautions, adverse events, and instructions prior to using this product.
IMPORTANT INFORMATION: These materials are intended to describe common clinical considerations and procedural steps for the use of referenced technologies but may not be appropriate for every patient or case.
Decisions surrounding patient care depend on the physician’s professional judgment in consideration of all available information for the individual case.
Boston Scientific does not promote or encourage the use of its devices outside their approved labeling. Case studies are not necessarily representative of clinical outcomes in all cases as individual results may vary.
Potential risks for Boston Scientific suburetheral slings: the following adverse events have been reported due to suburethral sling placement, any of which may be ongoing, but are not limited to: as with all implants, local irritation at the wound site and/or a foreign body response may occur, foreign body reaction may be acute or chronic, pain (pelvic, vaginal, groin/thigh, suprapubic, dyspareunia) (acute or chronic), dyspareunia, tissue responses to the mesh implant could include: erosion into organs (urethra, bladder or other surrounding tissues); exposure/extrusion into the vagina, mesh contact with urine via erosion/exposure/extrusion may result in stone formation, scarring/scar contracture, necrosis, fistula formation (acute or chronic), inflammation (acute or chronic), mesh contracture, tissue contracture, vaginal shortening or stenosis that may result in dyspareunia and/or sexual dysfunction, pain with intercourse that may not resolve, exposed mesh may cause pain or discomfort to the patient’s partner during intercourse, sexual dysfunction, including the inability to have intercourse. Like all foreign bodies, the mesh may potentiate an existing infection. Allergic reaction has been reported. Known risks of surgical procedures for the treatment of incontinence include: pain, ongoing pain (pelvic, vaginal, groin/thigh, suprapubic, dyspareunia), severe, chronic pain, apareunia, leg weakness, infection, de novo detrusor instability, complete failure of the procedure/failure to resolve a patient’s stress urinary incontinence, voiding dysfunction (incontinence, temporary or permanent lower urinary tract obstruction, difficulty urinating, pain with urination, overactive bladder, and retention), bruising, bleeding (vaginal, hematoma formation), abscess, vaginal discharge, dehiscence of vaginal incision, edema and erythema at the wound site, perforation or laceration of vessels, nerves, bladder, urethra or bowel may occur during placement. The following additional adverse events have been reported for the Solyx SIS system: dysuria, hematuria. The occurrence of these events may require surgical intervention and possible removal of the entire mesh. In some instances, these events may persist as a permanent condition after surgical intervention or other treatment. Removal of mesh or correction of mesh-related complications may involve multiple surgeries. Complete removal of mesh may not be possible and additional surgeries may not always fully correct the complications.
Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
Bench test study results may not necessarily be indicative of clinical performance.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician trained in use of surgical mesh repair of stress urinary incontinence. Refer to package insert provided with this product for complete indications for use, contraindications, warnings, precautions, adverse events, and instructions prior to using these products.
All images are the property of Boston Scientific.
All trademarks are the property of their respective owners.