WATCHMAN FLXTM
Left Atrial Appendage Closure Device
Push forward to the future of LAAC.
Confidently.
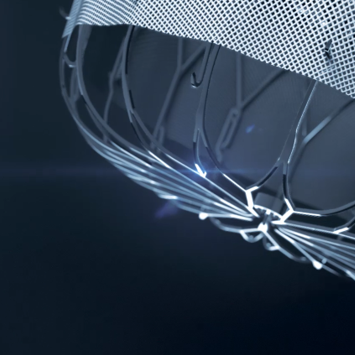
WATCHMAN FLX is the new generation LAAC device combining the experience of a clinically proven platform with a new, intuitive implantation technique for confident LAA closures of even challenging anatomies due to its excellent conformity to LAA anatomies.
Key Resources
Full control for an intuitive, safe and precise positioning
Enhanced conformability for confident closure
Minimal metal exposure for optimized healing
Watch the animation now
A journey of success

Join us on the FLX journey
Watch the video now
Flexibility to expand treatment options
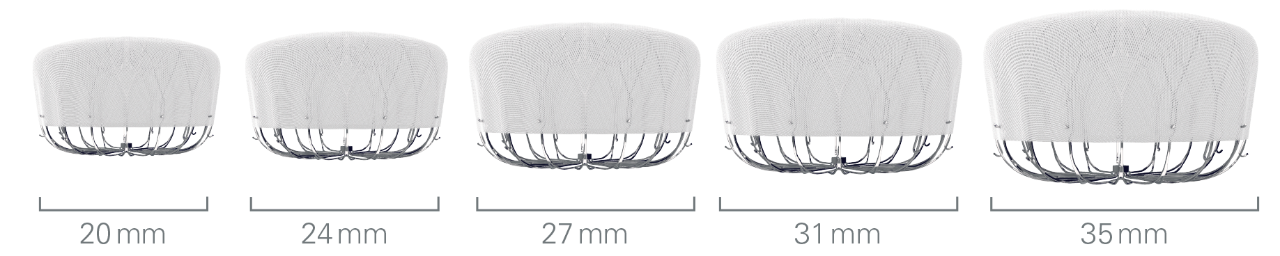
Product Information
| Description | Specifications |
| WATCHMAN FLX™ | Nitinol frame with Polyethylene Terephthalate (PET) with distal fluoroscopic marker |
| Description | Specifications |
| Sheath Material | Braided Pebax® with PTFE liner and platinum/iridium marker band |
| Description | Specifications |
| Hub | Material Pebax® with polycarbonate cap |
| Sheath Material | Pebax® with PTFE liner and platinum/iridium marker band |
| Dilator | HDPE/LDPE high density polyethylene/low density polyethylene (50:50 blend) |