ELUVIA™
Drug-Eluting Vascular Stent System
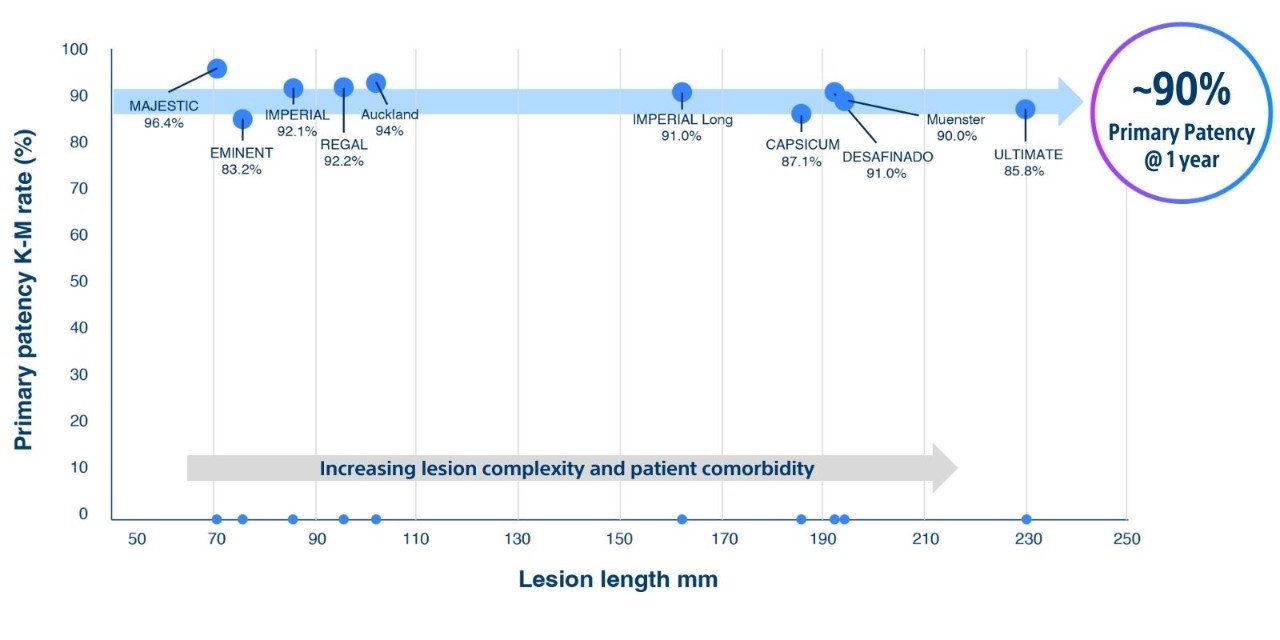
Eluvia™ is the unrivaled SFA stent – no matter the lesion complexity, no matter the patient. Restenosis presents a significant challenge in the management of superficial femoral artery (SFA) disease. Eluvia is specifically engineered for sustained drug release and efficient drug transfer, effectively overcoming challenges associated with peripheral arterial disease (PAD). It consistently delivers durable outcomes, even in the face of challenging SFA disease.
Key Resources
Product Details
Built for sustained drug release and efficient drug transfer.
Eluvia is designed to meet the challenges of the superficial femoral artery (SFA) with outstanding flexibility and precise stent placement. Only the Eluvia drug-eluting stent (DES) offers sustained drug release to match the restenosis process in the SFA, with the lowest drug dose density1 delivered by the most proven polymer.
Any lesion. Any patient. Zero doubt.
Eluvia™ size matrix

Eluvia™ Drug-Eluting Vascular Stent System UPN and GTIN Codes
| Description | UPN |
| ELUVIA EU 6X40 75 CM | H74939295600470 |
| ELUVIA EU 6X40 130 CM | H74939295600410 |
| ELUVIA EU 6X60 75 CM | H74939295600670 |
| ELUVIA EU 6X60 130 CM | H74939295600610 |
| ELUVIA EU 6X80 75 CM | H74939295600870 |
| ELUVIA EU 6X80 130 CM | H74939295600810 |
| ELUVIA EU 6X100 75 CM | H74939295601070 |
| ELUVIA EU 6X100 130 CM | H74939295601010 |
| ELUVIA EU 6X120 75 CM | H74939295601270 |
| ELUVIA EU 6X120 130 CM | H74939295601210 |
| ELUVIA EU 6X150 75 CM | H74939295601570 |
| ELUVIA EU 6X150 130 CM | H74939295601510 |
| ELUVIA EU 7X40 75 CM | H74939295700470 |
| ELUVIA EU 7X40 130 CM | H74939295700410 |
| ELUVIA EU 7X60 75 CM | H74939295700670 |
| ELUVIA EU 7X60 130 CM | H74939295700610 |
| ELUVIA EU 7X80 75 CM | H74939295700870 |
| ELUVIA EU 7X80 130 CM | H74939295700810 |
| ELUVIA EU 7X100 75 CM | H74939295701070 |
| ELUVIA EU 7X100 130 CM | H74939295701010 |
| ELUVIA EU 7X120 75 CM | H74939295701270 |
| ELUVIA EU 7X120 130 CM | H74939295701210 |
| ELUVIA EU 7X150 75 CM | H74939295701570 |
| ELUVIA EU 7X150 130 CM | H74939295701510 |