AGENT™
Paclitaxel Coated-PTCA Balloon Catheter
Background
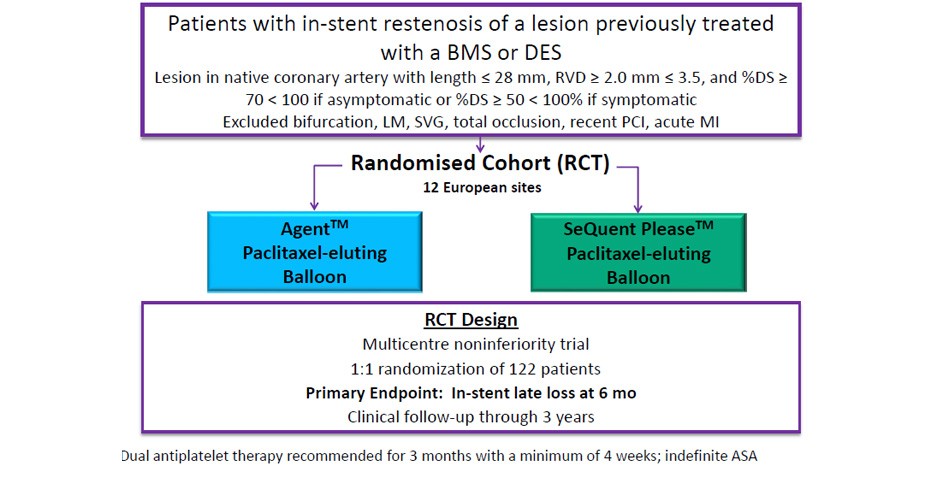
- Treatment of patients with in-stent restenosis (ISR) is an important clinical problem occurring in approximately 10% of PCI patients in real-world practice
- ISR is thought to result from aggressive neointimal proliferation or neoatherosclerosis
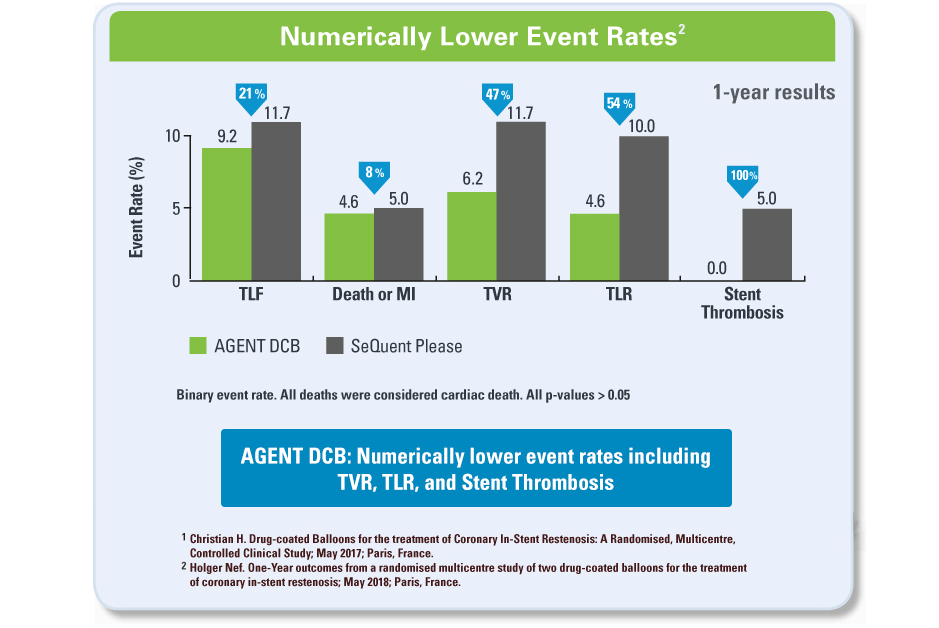
- Currently, drug-eluting stents and drug-coated balloons (DCB) are used to treat ISR
- DCB’s rapidly transfer the anti-proliferative drug, Paclitaxel, to the arterial vessel wall without adding another metal layer