EXALTTM Model D Single-Use Duodenoscope / Infection Prevention
Innovative, single-use design enhances safety
Despite adherence to rigorous disinfection and reprocessing protocols, multiple infection outbreaks worldwide have been linked to contaminated duodenoscopes used in endoscopic retrograde cholangiopancreatography (ERCP) procedures. As a result, the FDA has called for duodenoscopes with innovative designs to enhance safety, including scopes with disposable components or fully disposable duodenoscopes.1
The EXALTTM Model D Single-Use Duodenoscope completely eliminates the concern for device cross-contamination and the risk of patient-to-patient infection due to ineffective reprocessing.*
Complex cleaning processes increase post-ERCP infection risk
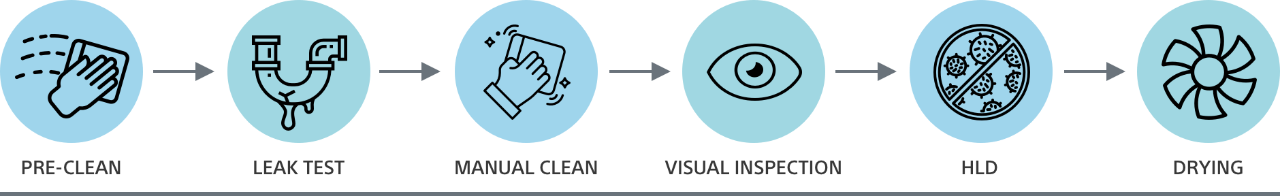
From pre-cleaning to drying, there can be more than 100 distinct steps to reprocessing a reusable duodenoscope – all of which introduce the opportunity for patient cross-contamination. Only a disposable duodenoscope like EXALT Model D eliminates the risks of cross-contamination associated with ineffective reprocessing.
FDA keeping a close eye on duodenoscope safetyThe FDA continues to closely scrutinize duodenoscope cross-contamination, with more safety communications issued on duodenoscopes than any other medical device since 2013. The FDA states that all hospitals and endoscopy facilities should transition to fully disposable duodenoscopes or those with disposable components to reduce the risk of patient infection – while recognizing that disposable scope components, like single-use endcaps, may lower but not eliminate infection risks.1 |
Single-use duodenoscopes vs. single-use endcaps
Clinical endoscopy technologies such as single-use endcaps and single-use duodenoscopes are designed to help reduce the risk of cross-contamination due to ineffective reprocessing. Yet, while disposable components like endcaps may lower the risks of infection, only single-use duodenoscopes – like EXALT Model D – completely eliminate post-ERCP infection risks as a result of cross-contamination from ineffective reprocessing.
| Benefit* | EXALT Model D | Single-Use Endcaps |
| Eliminates risk of patient infection due to ineffective reprocessing² | Yes | No |
| Eliminates duodenoscope reprocessing training and compliance³ | Yes | No |
| Decreases waste from reprocessing such as disinfecting consumables4 | Yes | No |
| Enhances infection prevention efforts aligning with 2022 FDA Safety Communication** | Yes | Yes |
*As compared to reusable duodenoscopes. Assumes full conversion of all ERCP procedures using reusable duodenoscopes to instead using the EXALT Model D Duodenoscope.

Find out how EXALT Model D may eliminate infection risk due to ineffective reprocessing and benefit your facility
Meta-analysis of reusable duodenoscope contamination5,6,7
A meta-analysis of 15 studies found that neither double high-level disinfection (HLD) nor ethylene oxide (EtO) gas sterilization eliminated the risk of contamination in reusable duodenoscopes that were considered patient ready.
![]()
15 studies
![]()
13,112 patient-ready duodenoscopes
![]()
15.3% contamination rate
FDA mandated surveillance studies6,7
Due to a growing concern over the post-ERCP infection risks with reusable duodenoscopes, the FDA mandated post-market surveillance studies to monitor the effectiveness of duodenoscope reprocessing.
Low-concern organisms
Up to 8.2% of properly collected samples tested positive for enough low-concern organisms to indicate a reprocessing failure.5,6

Fujifim 522 site: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=353&c_id=3725
Pentax: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=355&c_id=3727
Use Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication | FDA
![]()
Stay up to date
Sign up to receive periodic emails about EXALT Model D case studies, clinical data, reimbursement and more.
![]()
Connect with a rep
Request a rep to learn how EXALT Model D may help you address infection risks and improve patients’ lives.

