S-ICD™ System
Subcutaneous Implantable Defibrillator
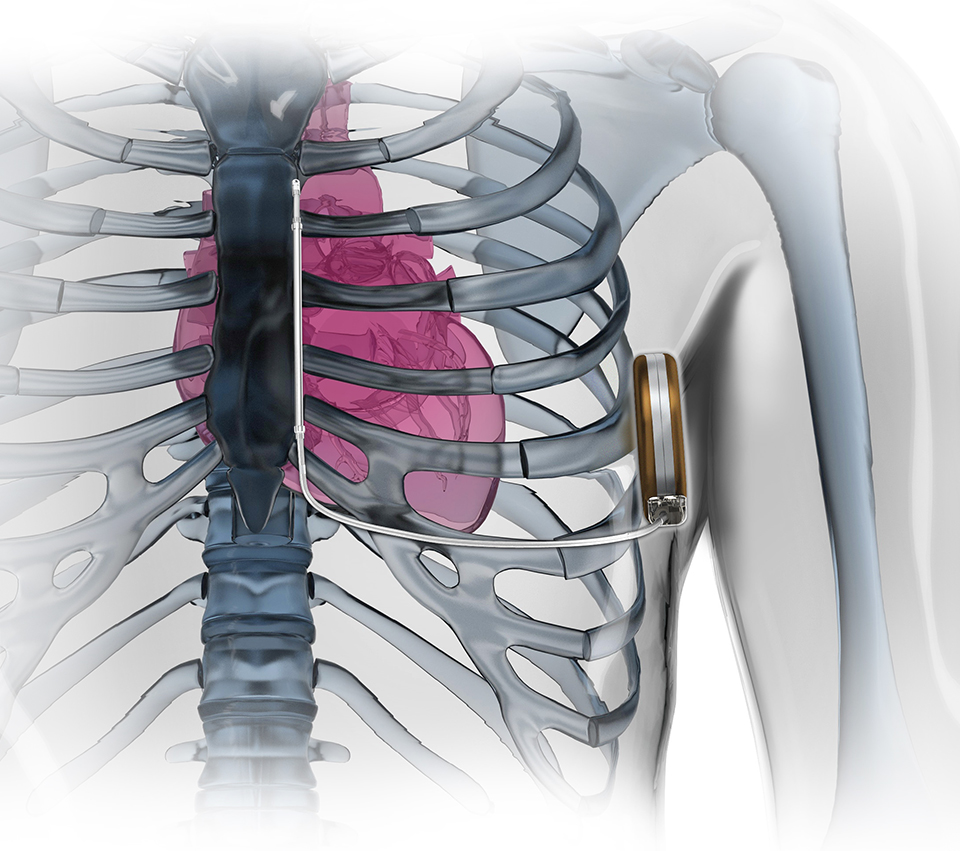
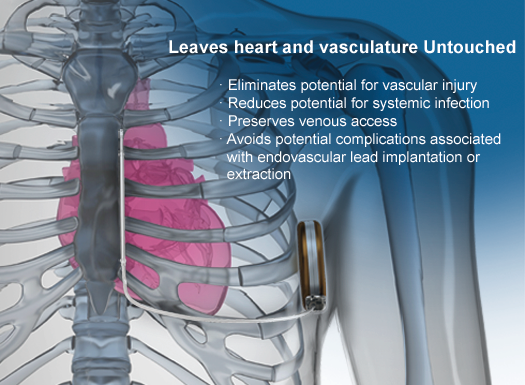
The S-ICD System is the world's first and only subcutaneous implantable defibrillator that provides protection from sudden cardiac arrest (SCA) while leaving the heart and vasculature untouched.
Explore
Protection without touching the heart
The S-ICD System is the world’s first and only subcutaneous implantable defibrillator that provides protection from sudden cardiac arrest (SCA) while leaving the heart and vasculature untouched. Like transvenous ICDs, the S-ICD System utilizes a pulse generator capable of delivering life-saving therapy. Unlike transvenous ICDs, the S-ICD System uses a subcutaneous electrode and analyzes the heart rhythm – rather than individual beats – to effectively sense, discriminate, and convert VT/VF. For years patients’ lives have been extended by implanting transvenous implantable defibrillators. Now the S-ICD System provides a new solution to protect patients from SCA, without touching the heart.
Effective defibrillation without transvenous leads
- Effective detection and conversion of induced and spontaneous VT/VF episodes1,2
- Low rates of significant clinical complications1
- Effective discrimination of AF and SVT from VT/VF1,2,3
- Rate of inappropriate therapy is consistent with transvenous ICDs1,2,3
Sophisticated technology
- Identifies and classifies the heart rhythm, rather than individual beats
- Revolutionary approach to sensing the subcutaneous signal
- INSIGHT™ algorithm effectively discriminates between treatable and other high-rate supra-ventricular events
- Designed to allow self-termination of non-sustained tachyarrhythmia
Durable subcutaneous electrode design
- Subcutaneous placement avoids intra-cardiac biomechanical stresses
- Multistrand cable-core design provides exceptional tensile strength
- Durable polyurethane body is highly resistant to abrasion
New Solution for a broad range of patients at risk for SC
- Effective for a majority of primary and secondary ICD candidates*
- Alternative for TV-ICD replacements due to lead malfunction/infection
- Ideal for primary electrical or structural heart disease
- Appropriate for a broad range of body types
Product Details
SQ-RX Pulse Generator Specifications
| Model Number | 1010 |
| Size (H x W x D) | 78.2 x 65.5 x 15.7 mm |
| Mass | 145 g |
| Volume | 69.9 cc |
| Longevity | Normal use*: 5 years |
| Electrode compatibility | Requires Q-TRAK subcutaneous electrode |
| Sensing configuration | Primary (ring to can), Secondary (tip to can), Alternate (tip to ring) Optimal sensing configuration automatically selected during Auto Setup (manual programming optional) |
| Gain selection | x1, x2 Optimal gain selection automatically selected during Auto Setup (manual programming optional) |
| Rhythm discrimination | INSIGHTᵀᴹ algorithm automatically activated when the Conditional Shock Zone is programmed |
| Shock polarity | Standard (coil to can), reserve (can to coil) Automatically selects and stores last successful shock polarity |
| Smart Chargeᵀᴹ | Shock polarity alters automatically after failed shock |
| Electrode compability | Automatically extends initial detection time to allow self termination of non-sustained tachyarrhythmias |
| Internal warning system | Audible tone alerts patient to elective replacement indicator, electrode impedance out of range, prolonged charge times, failed device integrity check |
| Shock Zone | 170 bpm - 250 bpm (steps of 10 bpm) |
| Conditional Shock Zone | Off, On 170 bpm - 240 bpm (minimum 10 bpm less than Shock Zone) |
| S-ICD System Therapy | Off, On |
| Post-shock pacing | Off, On (50 ppm, max 30 sec, demand-based) |
| Induction capability | 1-10 sec (50 Hz/200 mA) |
| Delivered Energy | 80J biphasic (only programmable during manual shock and induction test: 10J - 80J, steps of 5J) |
| Shocks per episode | Maximum of 5 shocks |
| Episode storage | S-ECG storage for over 40 arrythmic events (treated & untreated) |
| Other data | Electrode impedance System status (remaining battery life, patient alerts, etc.) Date and time stamp |
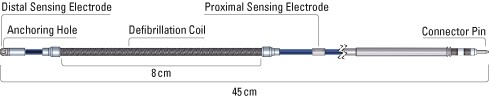
Q-Trak® Subcutaneous Electrode Specification
| Model Number | 3400 |
| Type | Tripolar |
| Length | 45 cm |
| Distal tip size (Diameter) | 12 Fr / 4 mm |
| Coil size (Diameter) | 9 Fr / 3 mm |
| Electrode shaft size (Diameter) | 7 Fr / 2.33 mm |
| Sensing surface area Distal Proximal | 46 mm 36 mm² |
| Sensing location Distal Proximal | Distal electrode tip 120 mm from tip |
| Defibrillation surface area | 750 mm² |
| Defibrillation location | 20 - 100 mm from tip |
| Materials | |
| Electrodes | Polyurethane |
| Conductors | MP35N |
| Connector Pin | MP35N |
| Suture Sleeve | Silicone |
Ordering Information
| 1010 | Pulse Generator |
| 3400 | Electrode |
| 3200 | Tablet Programmer |
| 4760 | Suture Sleeve |
| *3010 | Electrode - Cameron Health model number* |