mySCS™
A Personalized and Connected SCS Experience
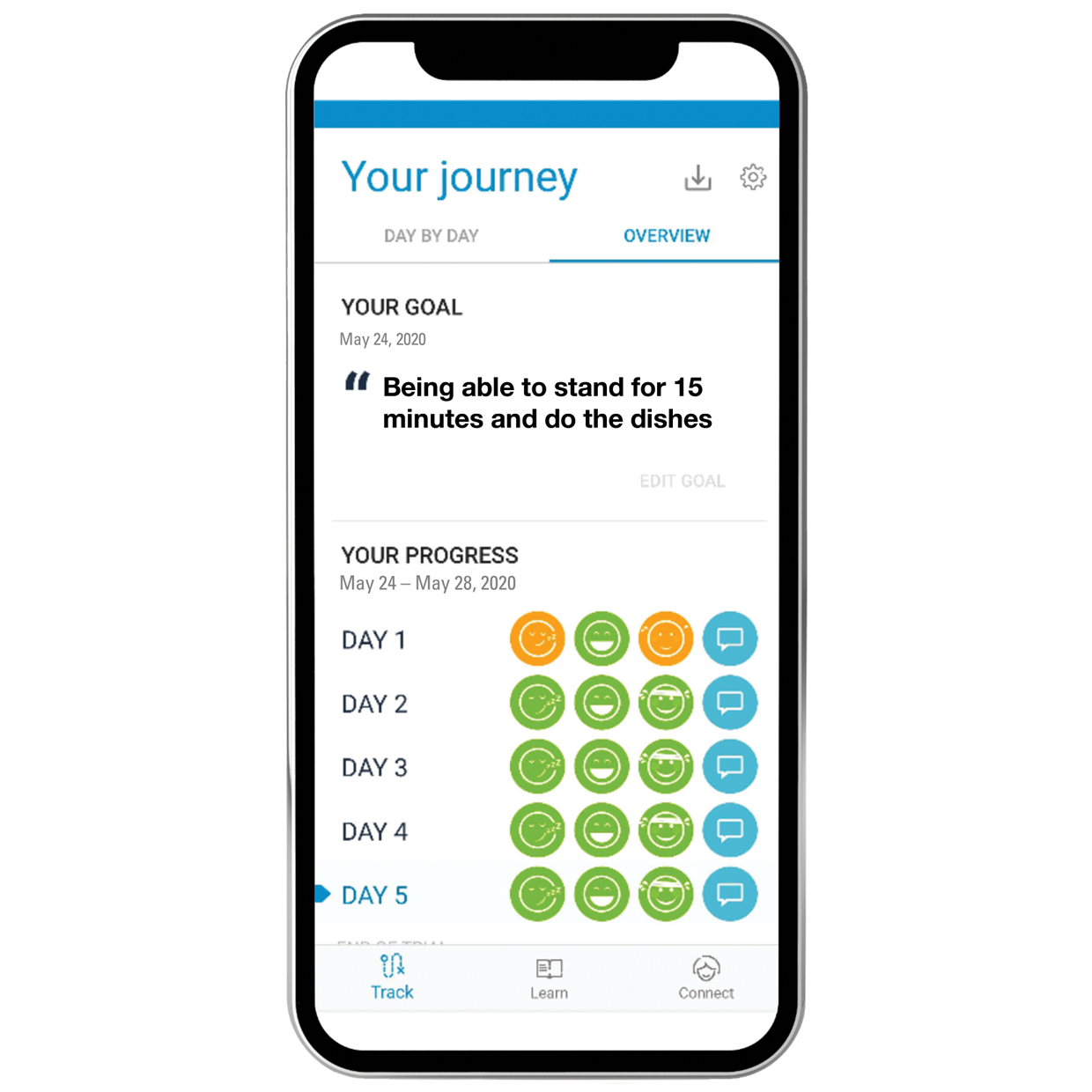
Patients using mySCS with a Boston Scientific device are more likely to have a successful trial1.
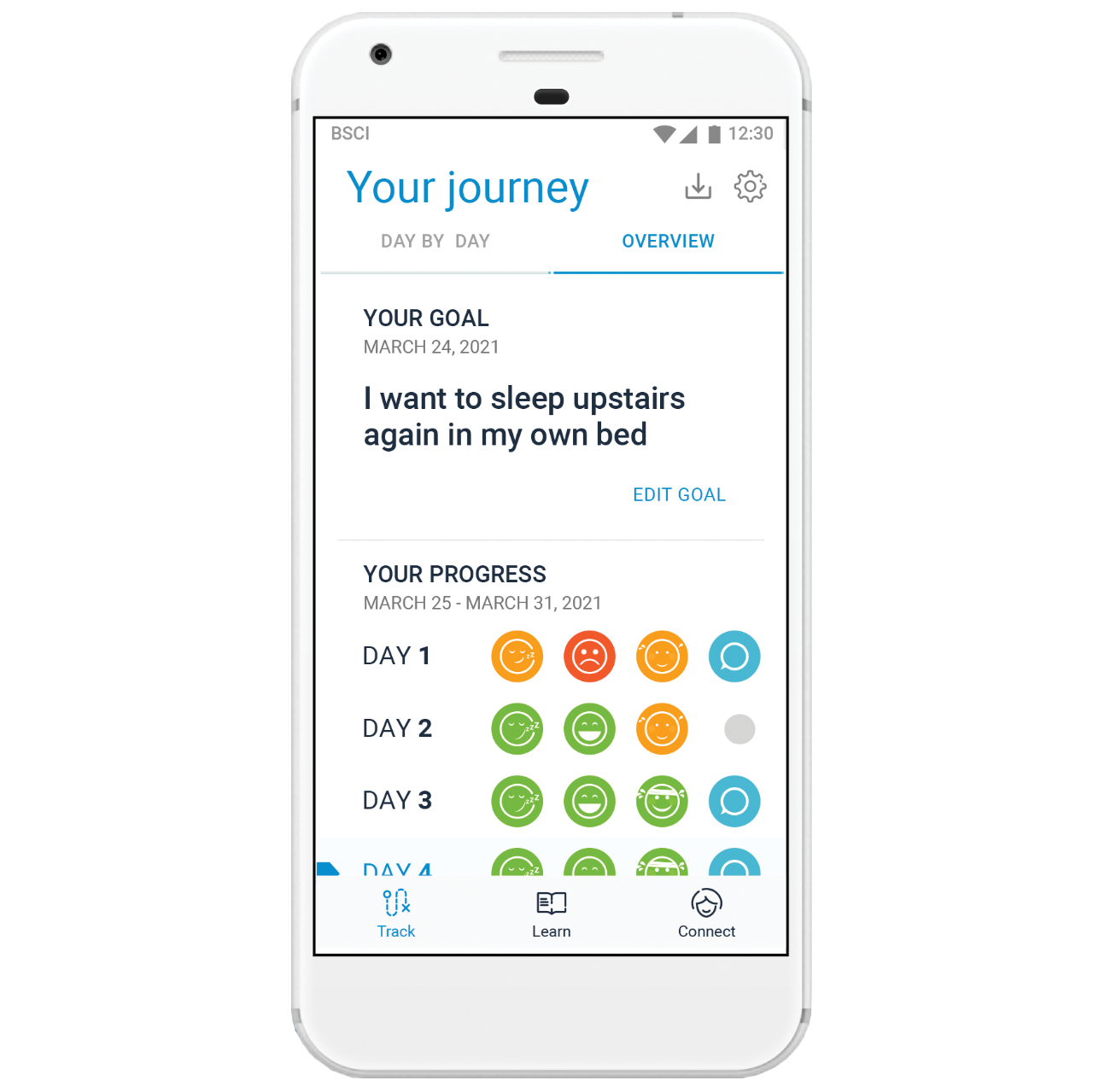
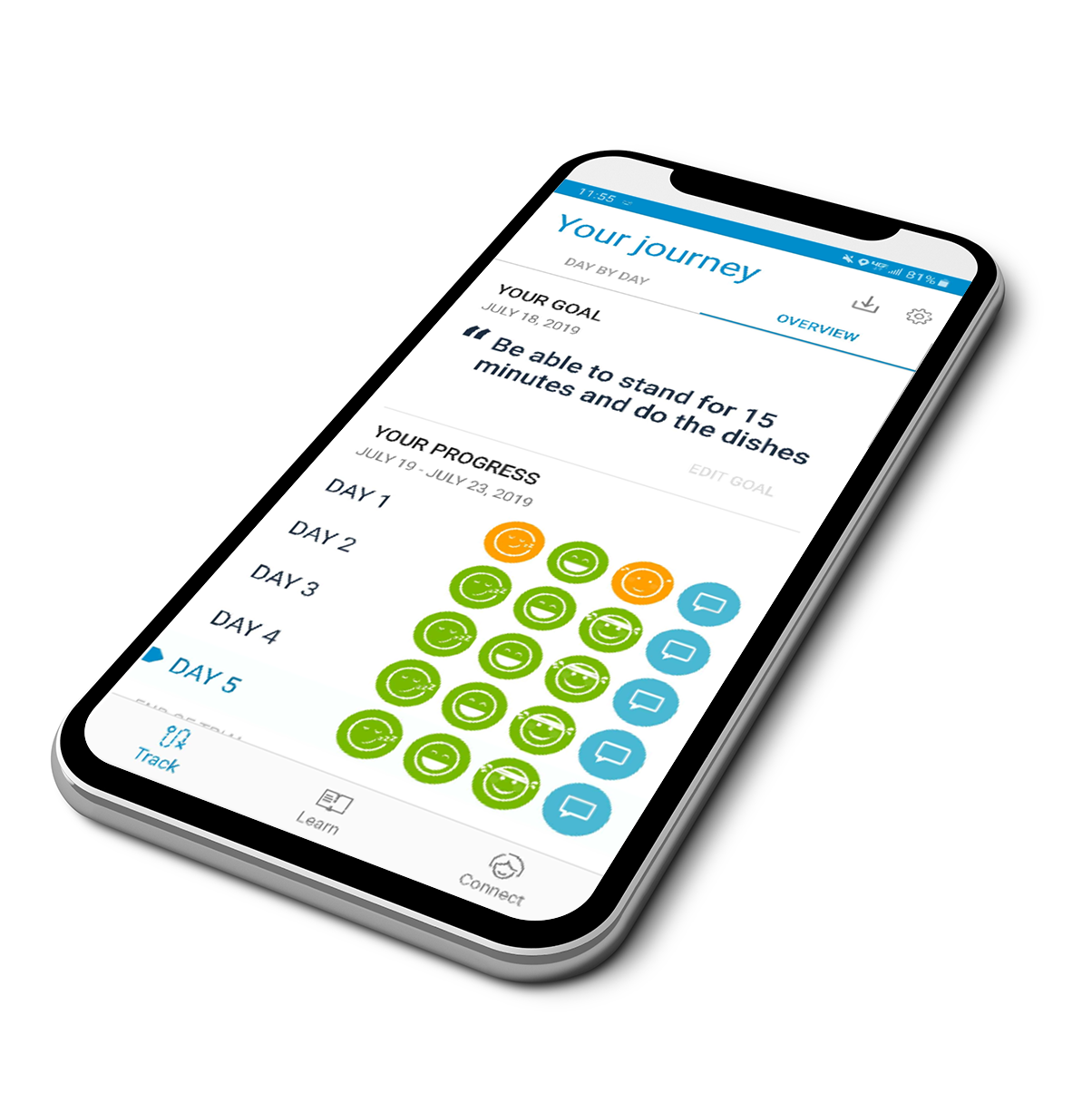
Boston Scientific can help you maximize your patients’ chances for successful therapy with mySCS™. It starts with patient education and personalized goal setting, followed by simple progress tracking and real-time expert support. In the end, it provides a trial summary report for documentation.
Key Resources
Have Your Patients Download mySCS
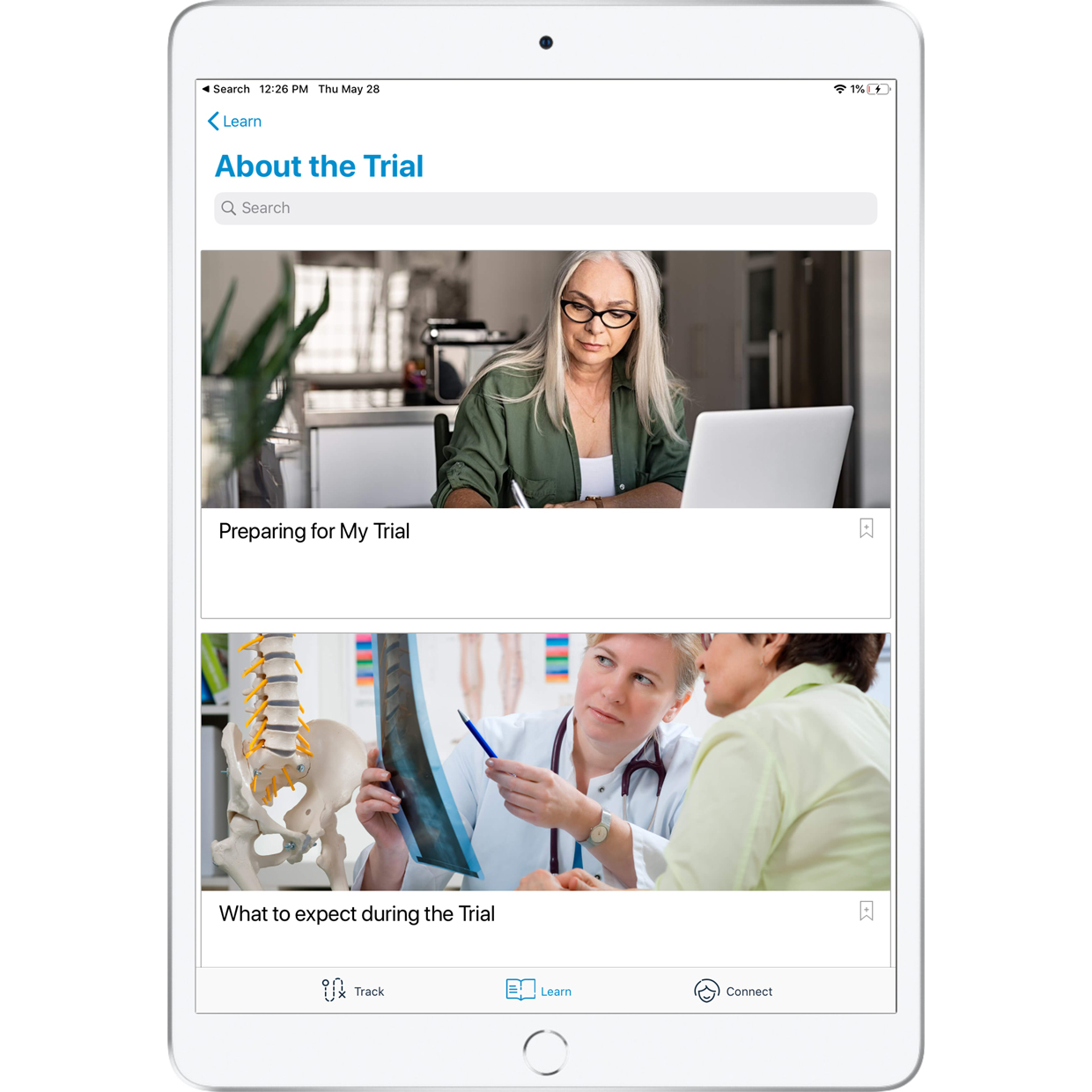
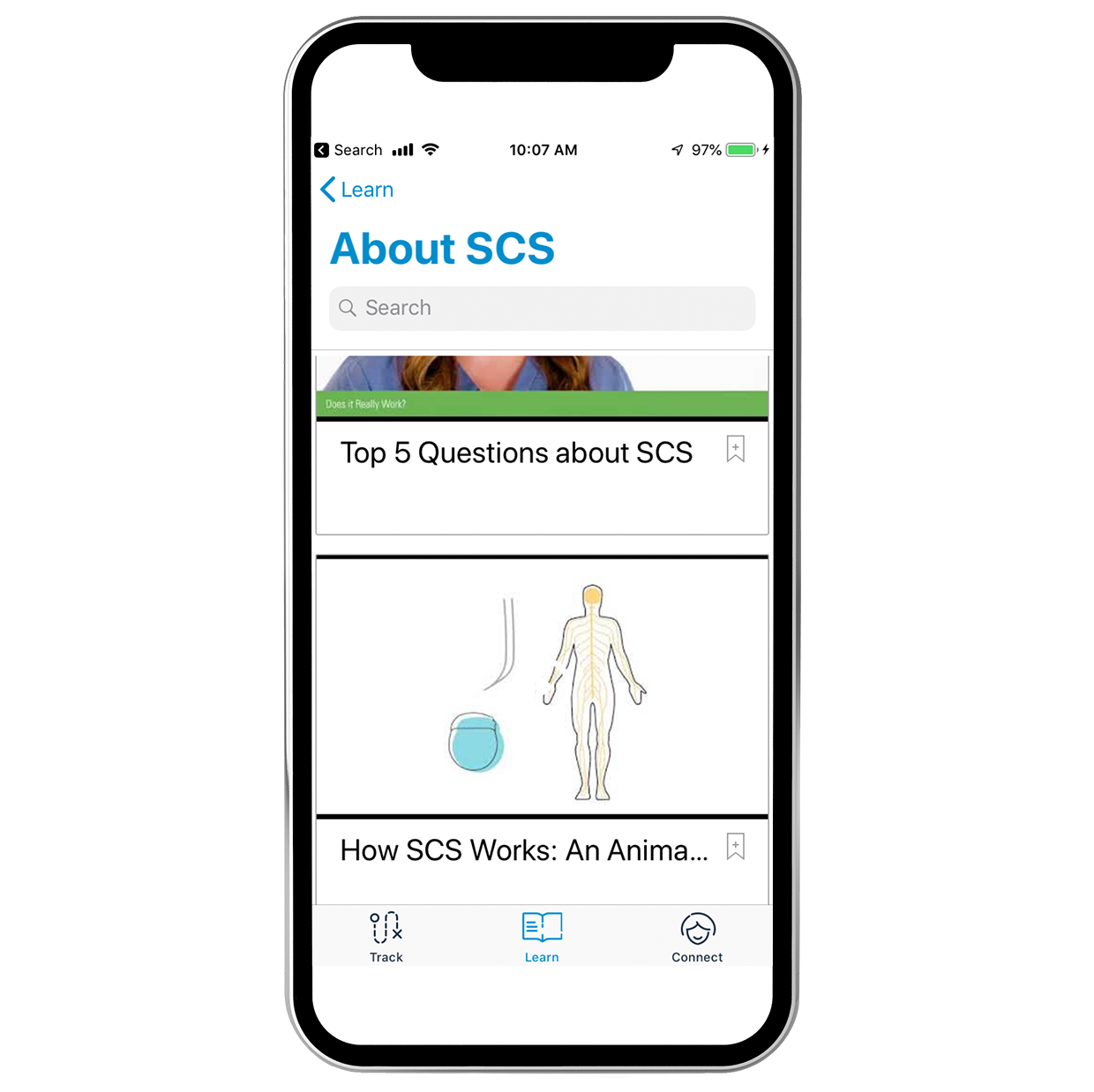
Give your patients access to learning resources and a direct connection with our SCS patient education team.
- Open the App Store on your mobile device
- Search for “mySCS”
- Select “mySCS App”
Discover New Features Now Available
- Expanded educational content
- SCS Journey Checklist
- Connect with a Patient Ambassador
- Spanish and Arabic translations
- Available on tablets and personal computers (in addition to mobile devices)
How mySCS Works
mySCS™ Simplifies the SCS Experience for More Consistent Outcomes
| Before Trial | During Trial | After Trial |
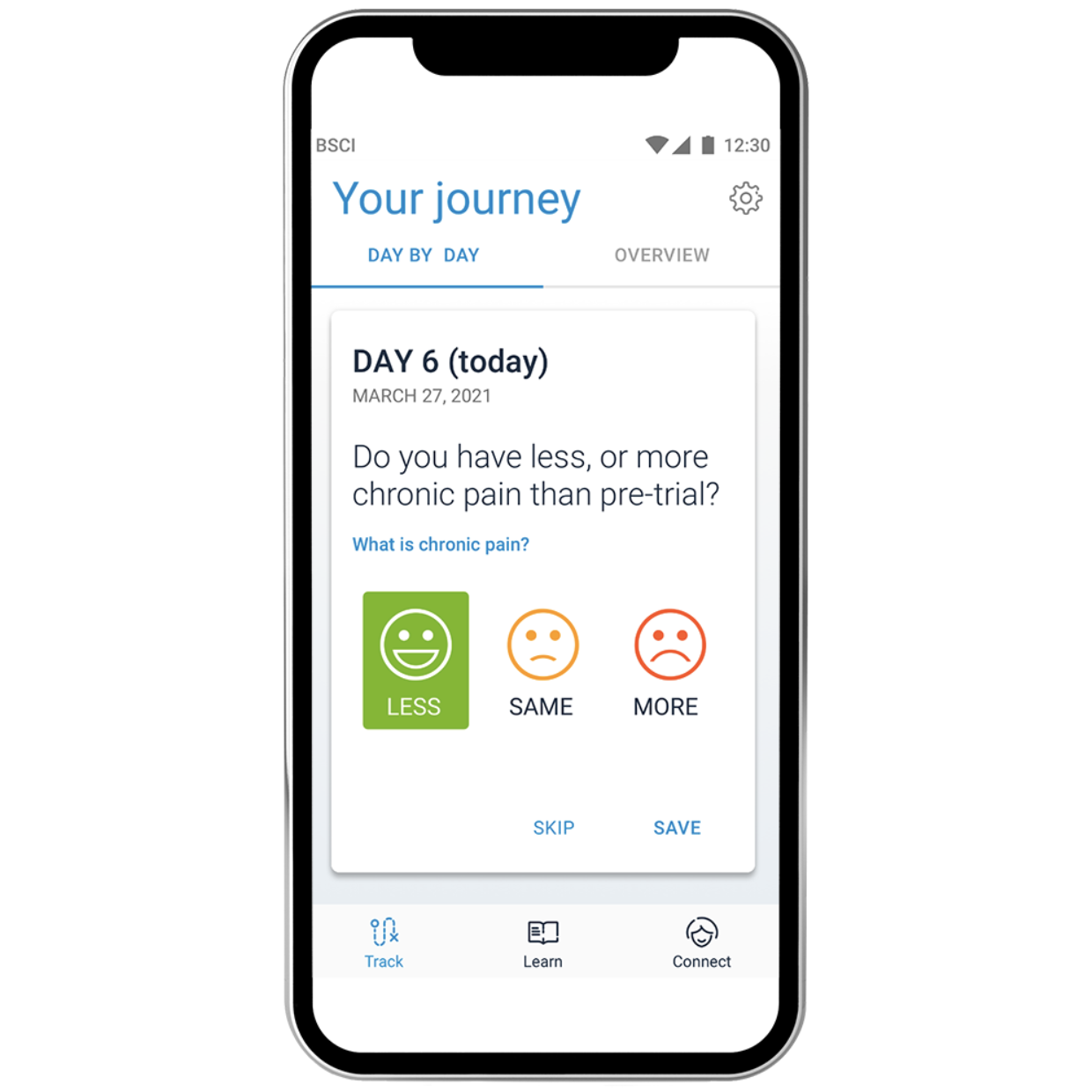
| Patient education resources for more clarity on the trial experience | Daily reminders to increase patient compliance | Detailed trial report to easily document success |
| Realistic goal setting to better assess trial outcome | Alerts for early communication when needed | Data-driven insights to optimize permanent implant |
Trial Summary Report