Study objective
To understand the impact of the EKOS Endovascular System low-dose, short treatment duration OPTALYSE PE study on various ultrasound-accelerated thrombolysis (USAT) protocols being used as the standard of care in the treatment of acute pulmonary embolism and associated long-term outcomes including quality of life outcomes.
489
489 patients in the prospective cohort
O ICH*
Zero intracerebral hemorrhagic events
23%
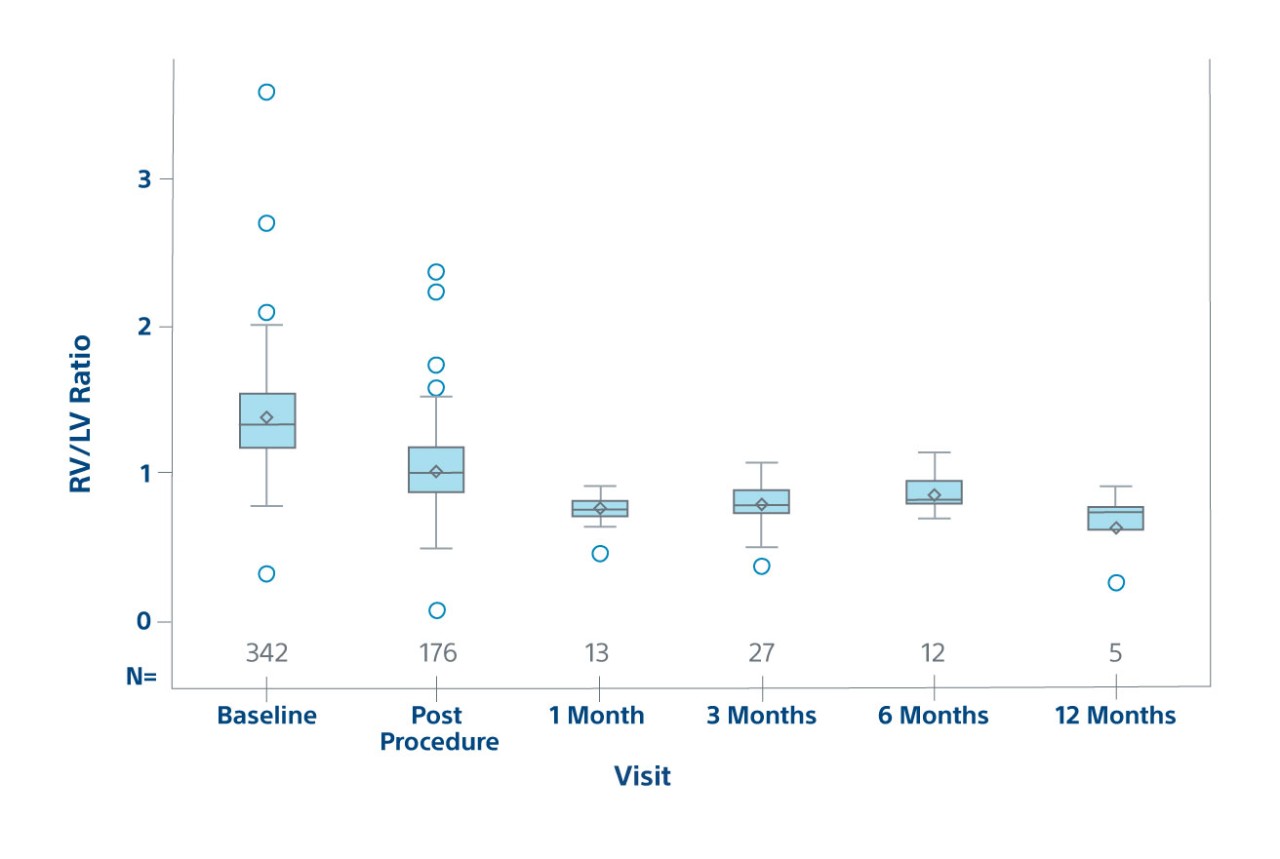
23% RV/LV reduction post-procedure
Results
Safety
Major bleeding at 72 hours | 8 (1.6) |
| Gastrointestinal hemorrhage | 2 (0.4) |
| Head laceration | 1 (0.2) |
| Subdural hematoma (pre-existing) | 1 (0.2) |
| Vascular access site hematoma | 4 (0.8) |
Recurrent VTE Within 30 Days | |
| Pulmonary Embolism | 2 (0.4) |
* 1 preexisting subdural hemorrhage (SDH)
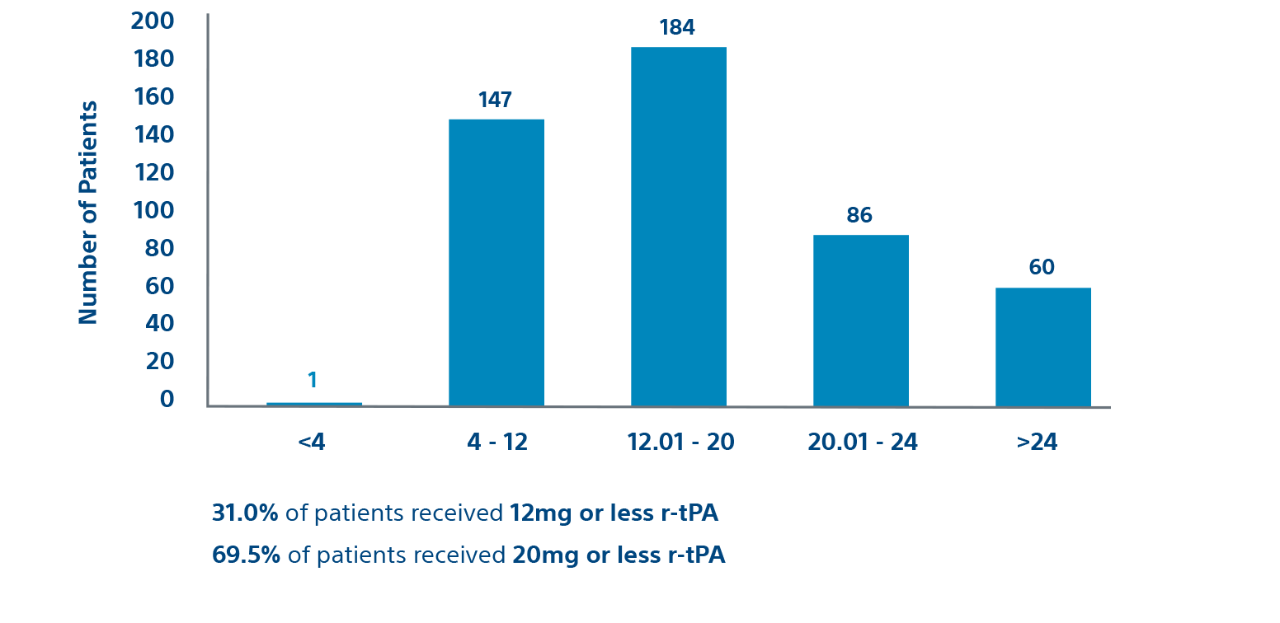
Procedural
Prospective cohort
Efficacy
RV/LV Ratio by Visit on ECHO
Efficacy population in prospective patients
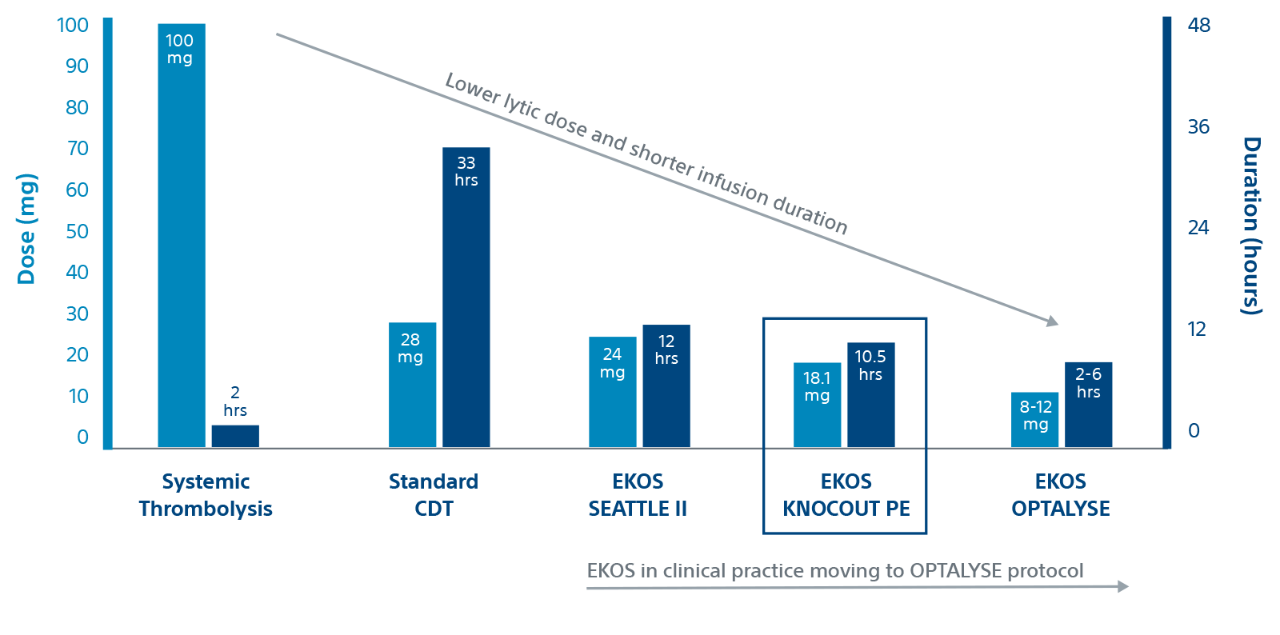
Evidence
KNOCOUT shows that contemporary clinical practices are moving to low-dose, short duration OPTALYSE protocols. It adds to the growing evidence that EKOS is effective at treating intermediate-risk and high-risk PE with lower lytic doses and shorter infusion durations compared to other thrombolytic therapies.
Duration and dose by therapy
References
1. KonstantinidesS, GeibelA, HeuselG, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N EnglJ Med. 2002;347:1143–1150.
2. Kuo W et al. CHEST 2015; 148(3):667 673.
3. Piazza G et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism. The SEATTLE II Study. J Amer Coll Cardiology: Cardiovasc Interventions 2015; 8(10):1382-1392.
4. Sterling KM et al. Prospective Multicenter International Registry of Ultrasound-Facilitated Catheter-Directed Thrombolysis in Intermediate-High and High-Risk Pulmonary Embolism (KNOCOUT PE). Circ Cardiovasc Interv. 2024;17:e013448. doi: 10.1161/CIRCINTERVENTIONS.123.013448
5. TapsonVF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11:1401-1410. doi: 10.1016/j.jcin.2018.04.008
