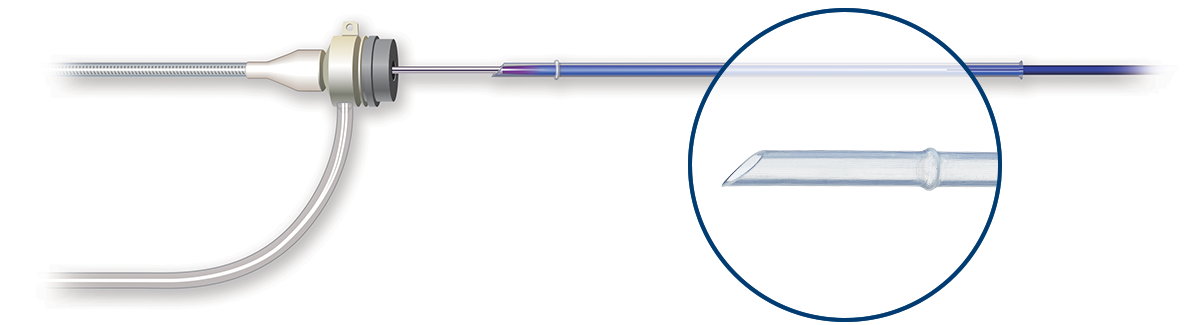
The lowest tip entry profile of any SFA DCB
Ranger DCB's low profile and tapered tip2 enables a smooth delivery with excellent trackability and navigation through alternative access3 sites.

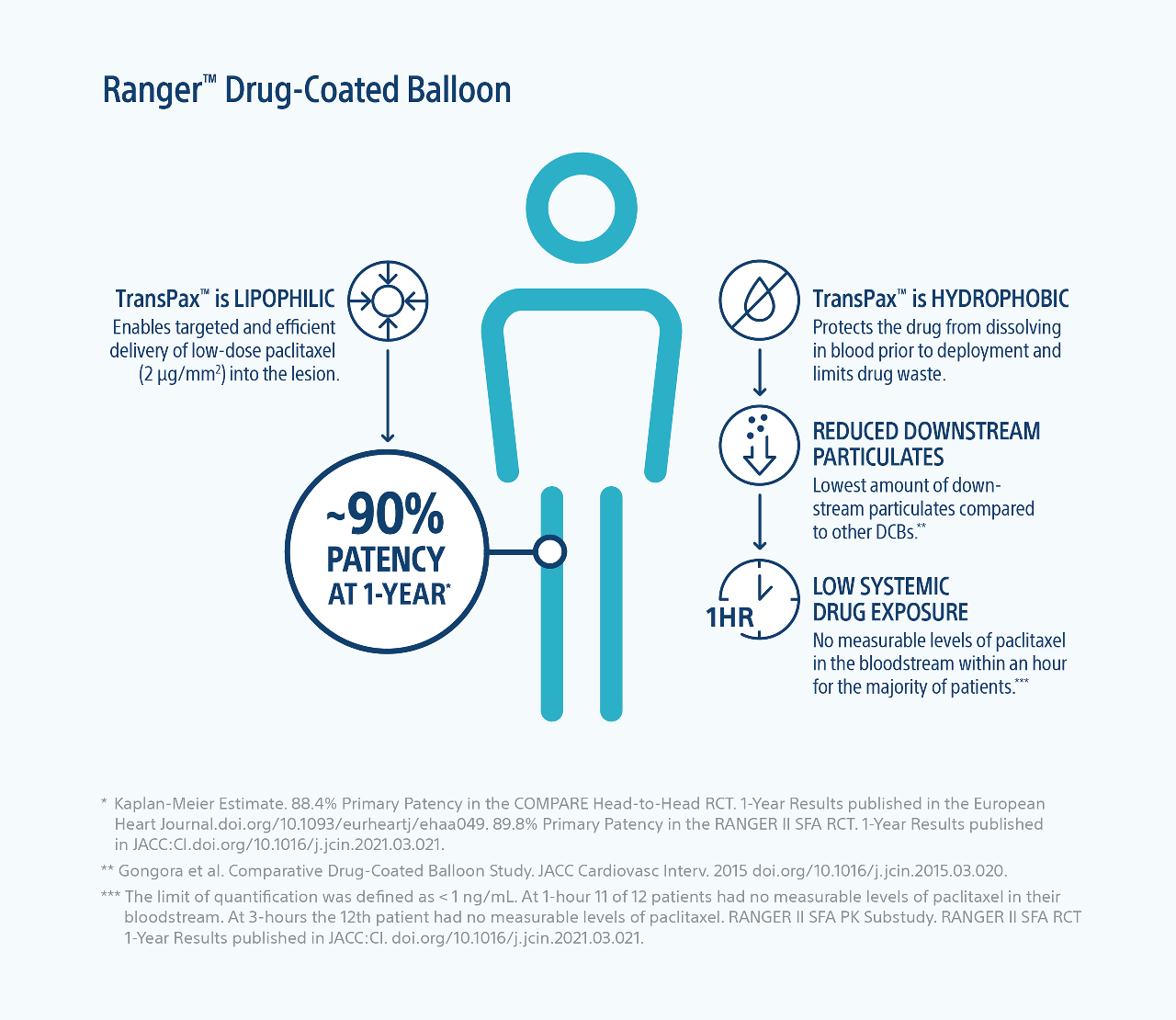
Efficient drug transfer
TransPax™ (citrate ester + low dose paclitaxel) is a next-generation coating that enables highly efficient drug transfer into the lesion.
Ranger DCB product details
Ranger DCB has a comprehensive matrix and is compatible with pedal access3
| Ranger matrix | 40 mm | 60 mm | 80 mm | 100 mm | 120 mm | 150 mm | 200 mm |
| 4 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 5 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 6 mm | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F | 5 F |
| 7 mm | 6 F | 6 F | 6 F | 6 F | 6 F | 6 F | 6 F |
Proprietary loading tool
Serves as the balloon and drug protector to help prevent drug loss during insertion and limit a physician’s exposure to the drug.