
Clinical Results
EMINENT Randomized Clinical Trial Results1
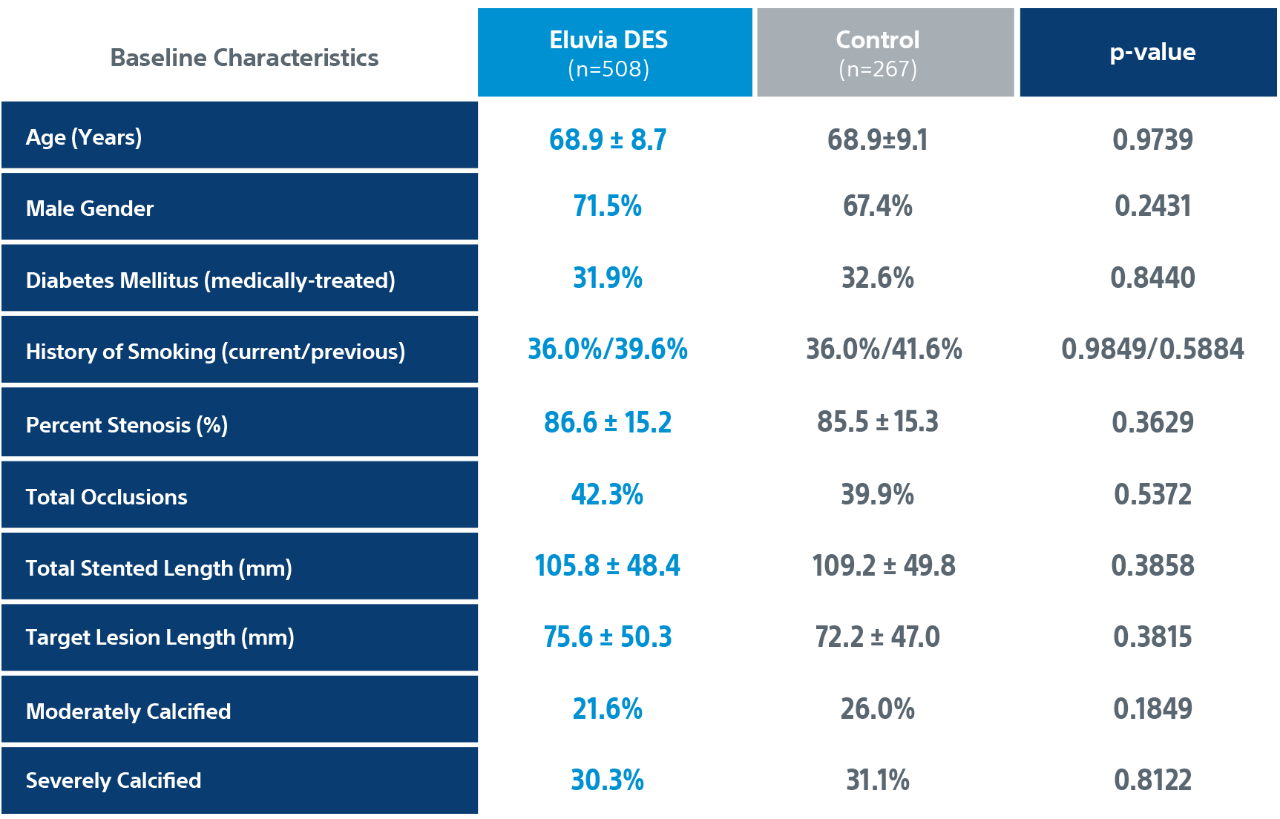
EMINENT is the largest RANDOMIZED CONTROLLED TRIAL (2:1) comparing Eluvia™ Drug-Eluting Vascular Stent System to self-expanding bare metal stents (BMS) for SFA/PPA EU multi-center; superiority trial; core lab adjudicated.
Eminent RCT 1-Year Primary Patency Results1
Eluvia demonstrated superiority over BMS1 with a statistically significant primary patency of 85.4% versus 76.3% through 1-Year.
**Log rand p-value compares the entire K-M curves from time point zero to day 390 (full 1-Year follow-up window)
ELUVIA DEMONSTRATED SUSTAINED CLINICAL IMPROVEMENT1
Eluvia demonstrated a statistically significant greater rate of sustained clinical improvement without reintervention over BMS through 1-Year.
EMINENT Randomized Controlled Trial Details
**Log-rank p-value compares the entire K-M curves from time point zero to day 390 (full 1-year follow-up window)
***In EMINENT, primary sustained clinical improvement was defined as an improvement (decrease) by at least 1 Rutherford category, without TLR.
1. EMINENT Clinical Trial 1-Year results presented by Professor Yann Goueffic, MD. VIVA 2021
2. Gray WA, et al; A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. 1-Year Results. The Lancet. 2018 Oct 27;392(10157):1541-1551. doi: 10.1016/S0140-6736(18)32262-1. Epub 2018 Sep 24. PMID: 30262332. .
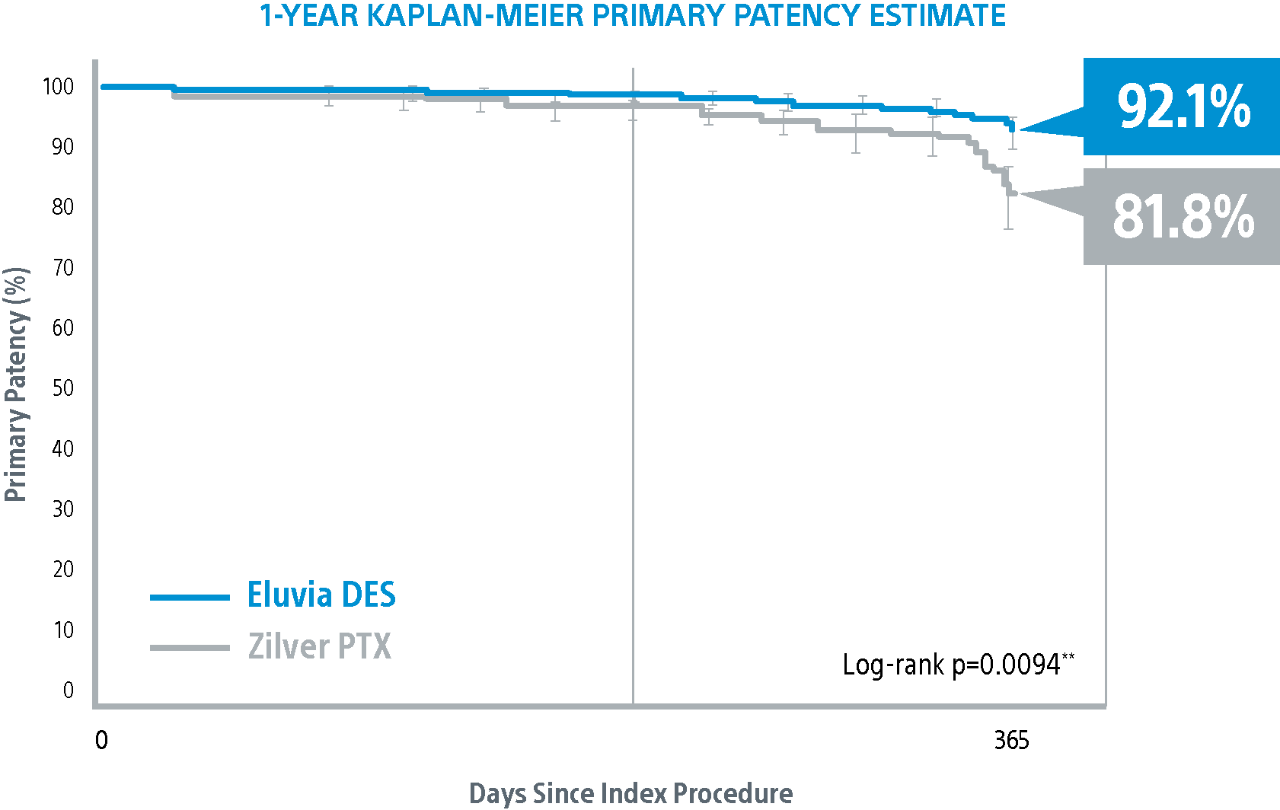
IMPERIAL Randomized Controlled Trial Results1,2
Eluvia DES demonstrated statistically significant difference in primary patency versus Zilver PTX and achieved the highest primary patency reported in any SFA DE Pivotal Trial at 1-Year3.
IMPERIAL RCT 1-Year Primary Patency Results*
Eluvia demonstrated superiority over BMS1 with a statistically significant primary patency of 85.4% versus 76.3% through 1-Year.
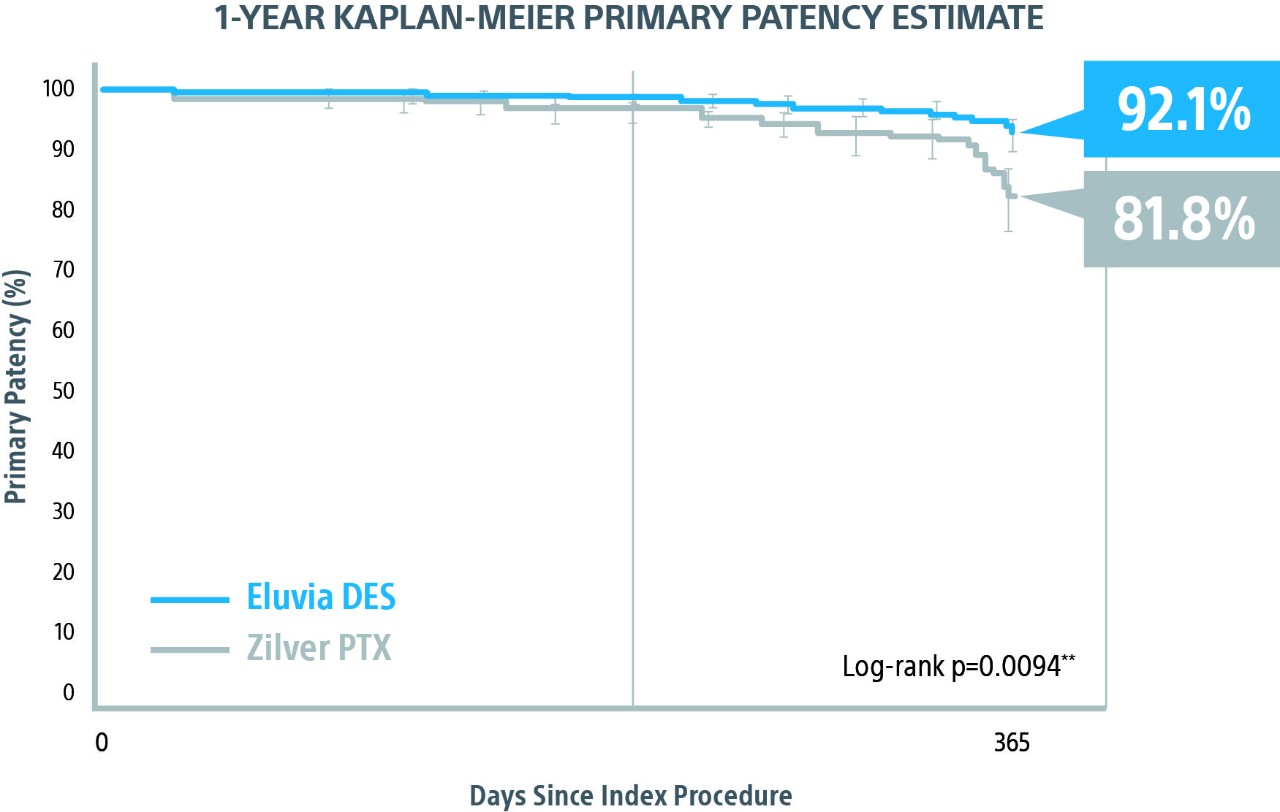
** Log-rank p-value compares the entire K-M curves from time zero to full 1-Year follow-up window.
IMPERIAL RCT 2-Year Primary Patency Results*
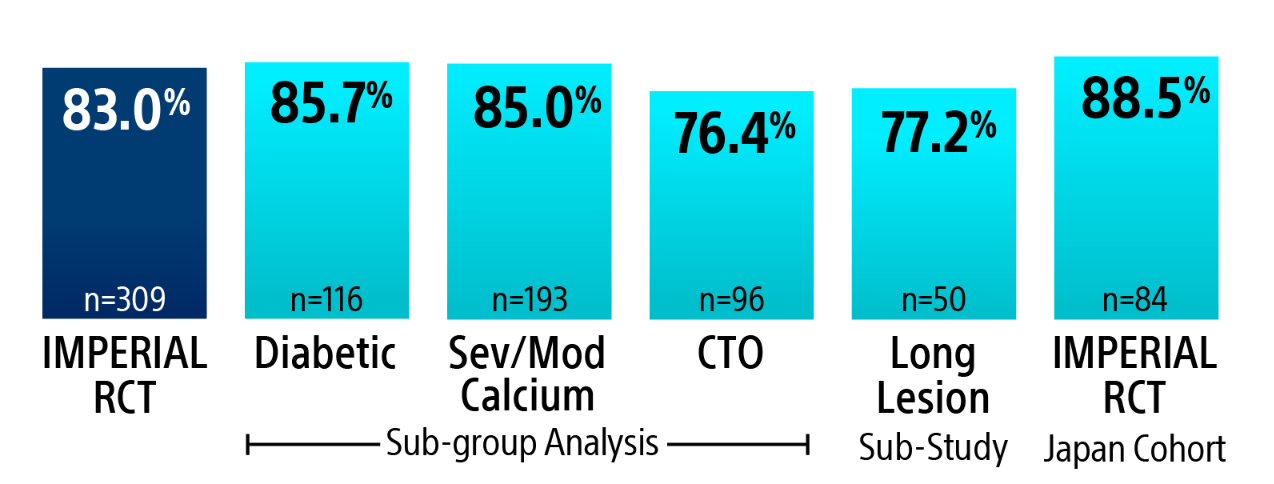
*** Vermassen, F. VIVA Late-Breaking Clinical Trials June 2020.
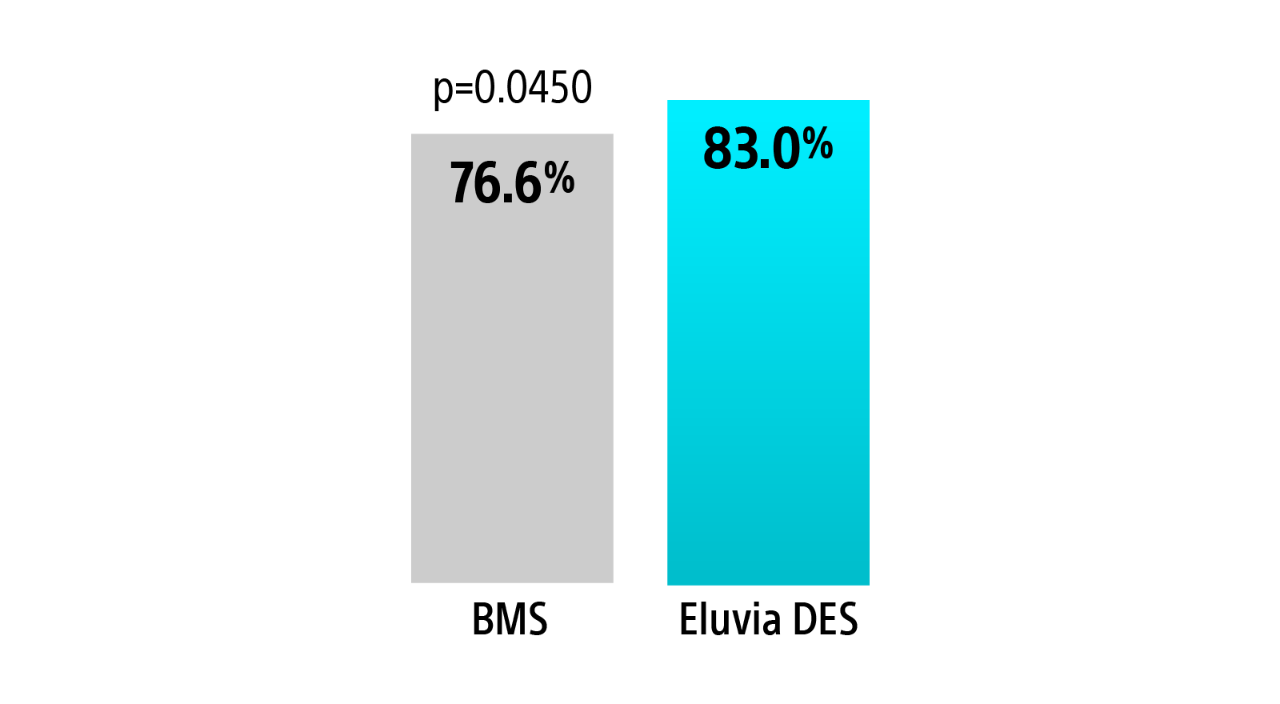
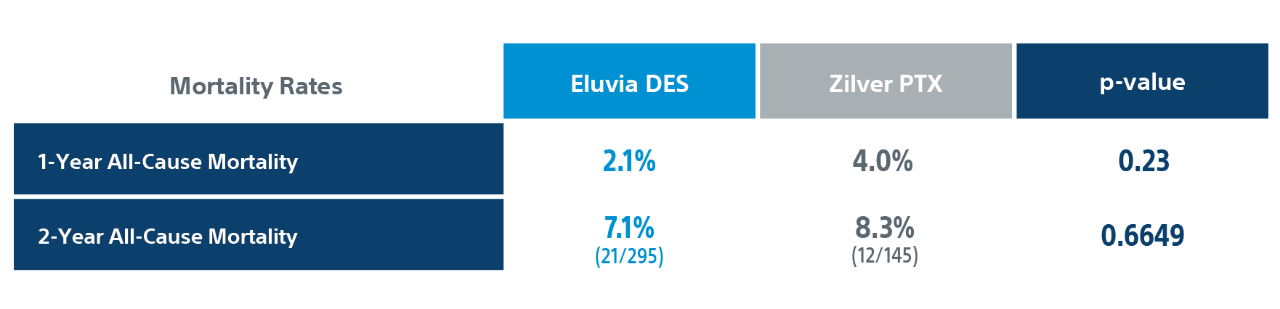
2-Year Clinically-Driven TLR4
Eluvia DES had statistically significant fewer CD-TLR’s compared to Zilver PTX at 2-Years.
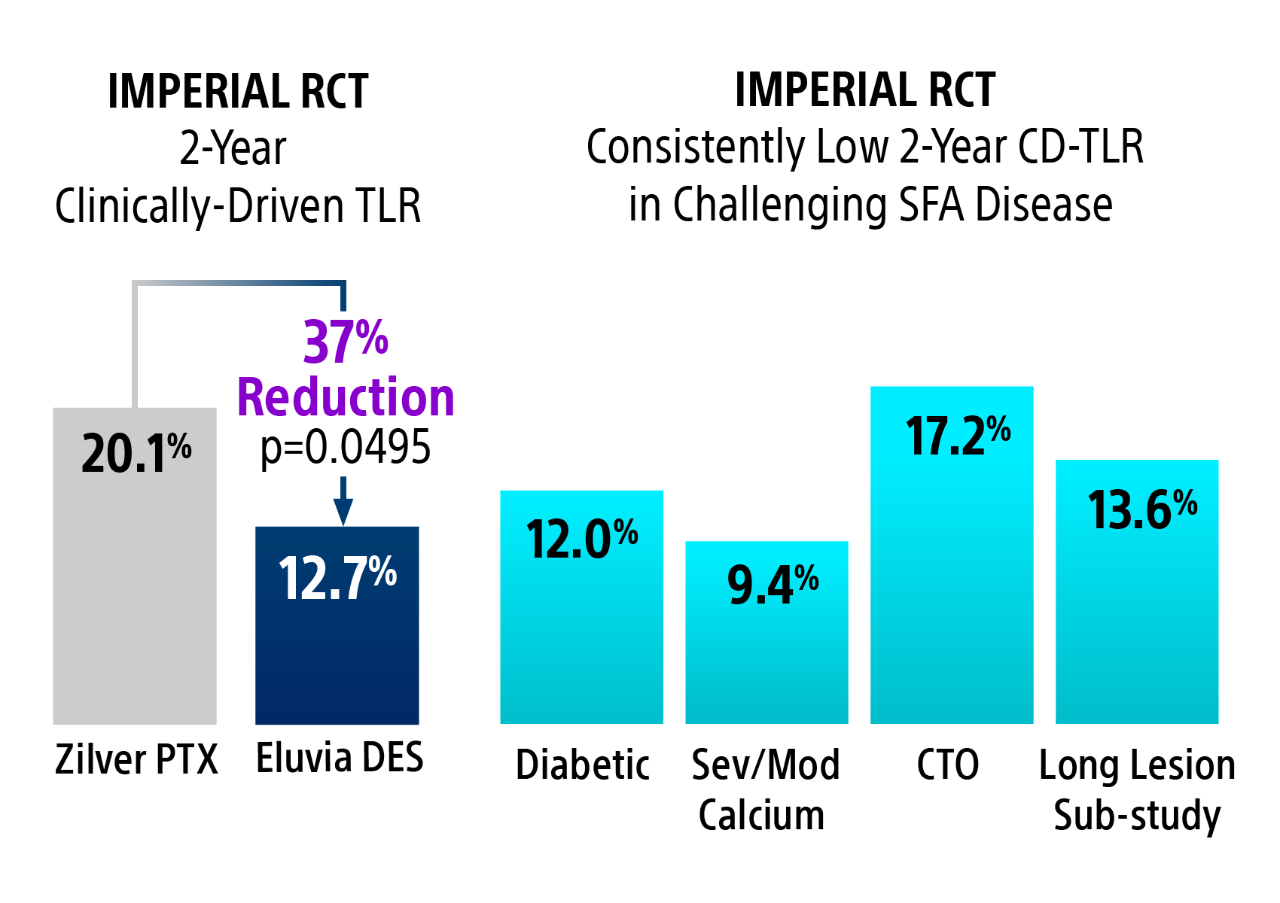
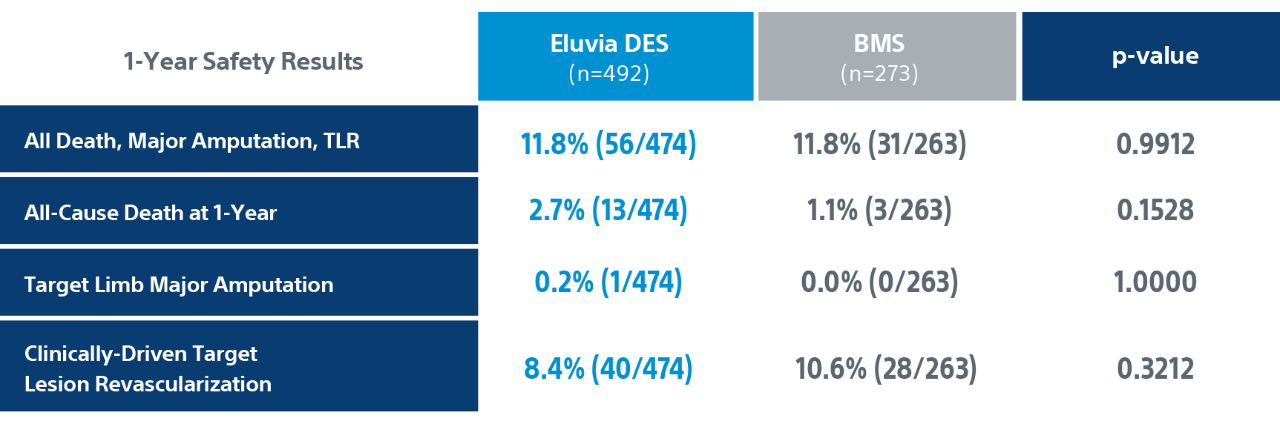
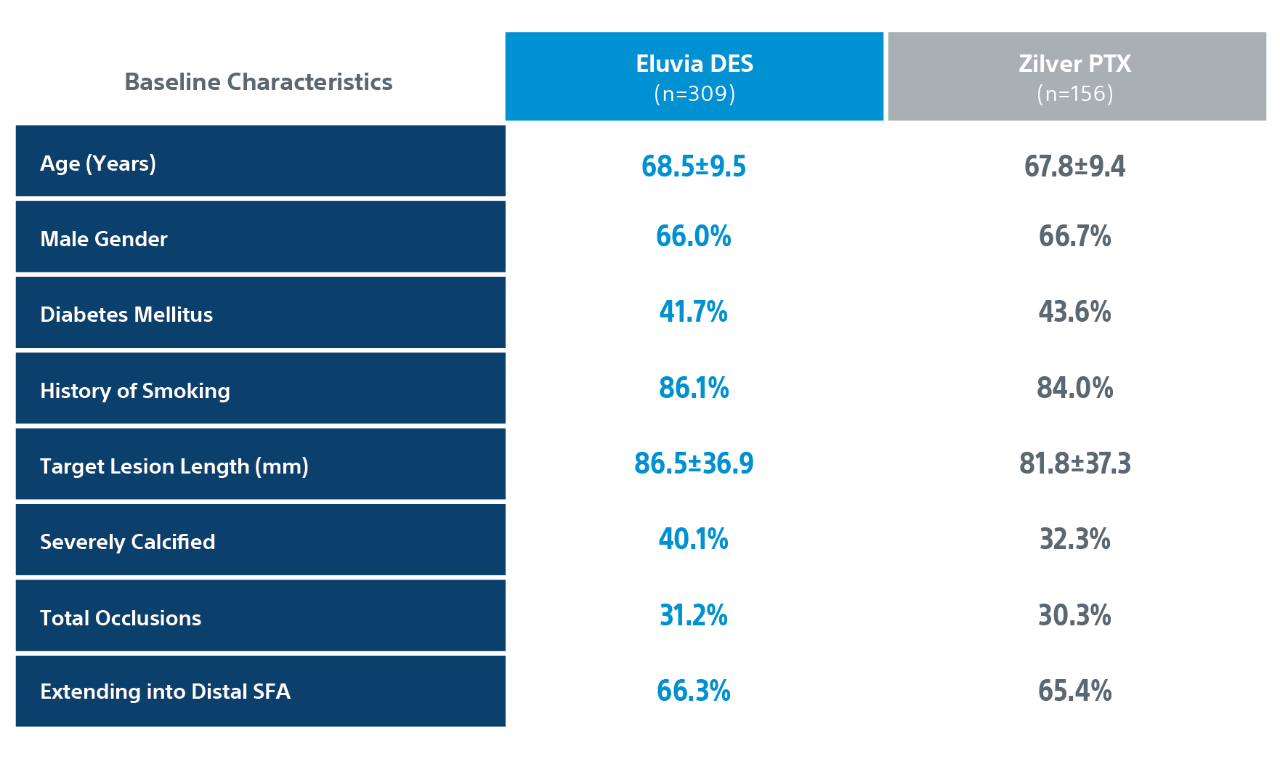
IMPERIAL Randomized Controlled Trial Details
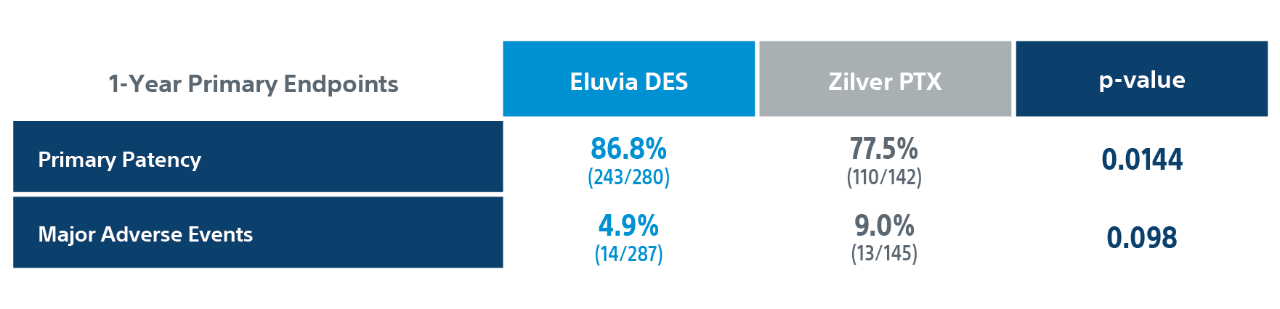
2. Kaplan-Meier Primary Patency Estimate through 1-Year (including follow-up window) was statistically significant with a p-value of 0.0094.
3. Based on 1-Year Kaplan-Meier estimates reported for IMPERIAL, RANGER II SFA, IN.PACT SFA, ILLUMENATE, LEVANT II and Primary Randomization for Zilver PTX RCT.
4. Long Lesion TLR data is as-treated as presented at FDA panel 2019. All other data sets are intention to treat, adapted from Gary, W. LINC 2020 presentation.
Explore DE Resources
Get physician perspectives, clinical data sheets and other resources about drug-eluting innovations.
Stay Up to Date
Receive emails about the latest drug-eluting technology news, innovations and events in your area.
















