2-year Results from a Pooled Analysis of the IDE Study and EFFORTLESS Registry
Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-year Results from a Pooled Analysis of the IDE Study and EFFORTLESS Registry
Background
The entirely subcutaneous implantable defibrillator (S-ICD™) is an alternative to transvenous defibrillators for the prevention of sudden cardiac death. Two studies have been undertaken to track the initial worldwide experience with the S-ICD. The EFFORTLESS Registry began enrollment in 2009 and continues to collect demographic, safety and efficacy data. The S-ICD System Clinical Investigation (IDE study) began enrolling in 2009 collecting the same information. The purpose of both studies was to evaluate the safety and efficacy of the S-ICD. The six month to one year data for each of these trials has been reported separately demonstrating safety and efficacy of the S-ICD.
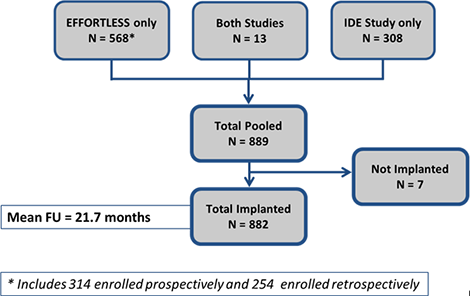
Combining these two studies provides a unique opportunity to evaluate complications as well as amass a significant number of spontaneous events in 882 implanted patients followed for 1,517 patient-years.
Demographics
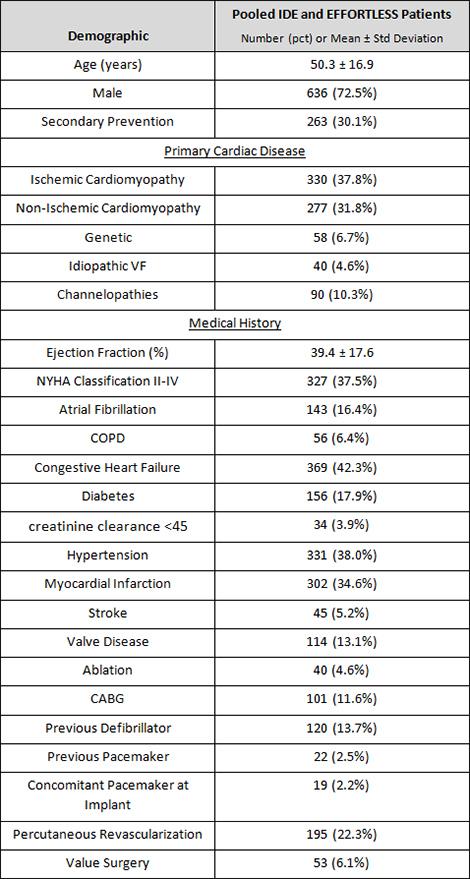
The trials enrolled patients meeting indications for an ICD, and excluded patients with documented spontaneous and frequently recurring ventricular tachycardia (VT) reliably terminated with anti-tachycardia pacing, as well as patients with unipolar pacemakers.
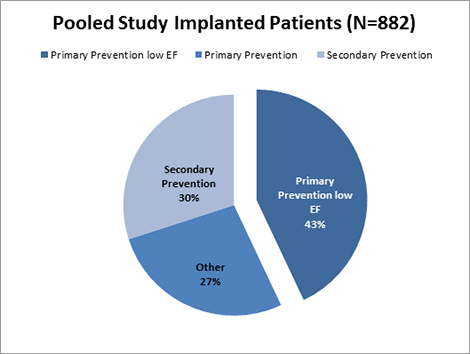
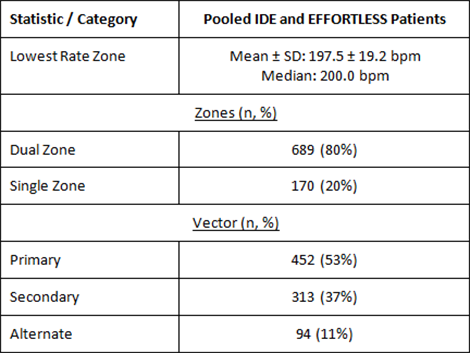
Primary prevention patients with ejection fraction ≤ 35 accounted for 43% of the study population, and together with secondary prevention patients (N=263, mean EF= 30%) made up the majority of implanted patients.
Trial Results
The Pooled Analysis of EFFORTLESS and IDE below have been published in the peer-reviewed Journal of American College of Cardiology (JACC). There were 882 patients implanted and followed for 651±345 days (1571.5 patient-years).
Therapy Efficacy
The spontaneous VT/VF efficacy was comparable to transvenous spontaneous event conversion at 90.1% successful first shock efficacy for discrete events, and 98.2% (109/111) efficacy with all available shocks.
Of two “unconverted” episodes
- One spontaneously terminated after the 5th shock
- In the other episode, the device prematurely declared the episode ended. A new episode was immediately reinitiated and the VF was successfully terminated with one shock
Complications
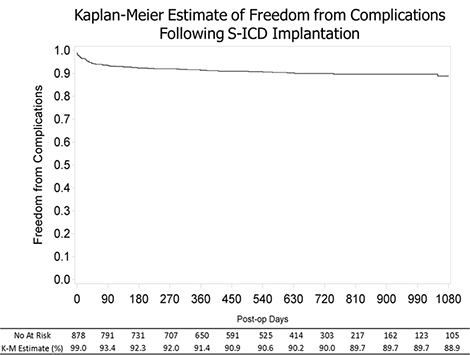
4.5% of patients experienced a complication within 30 days and 11.1% of patients over 3 years. There were no endovascular infections or electrode failures.
Extractions for pacing occurred in 0.4% of patients.
| Reason | Number (Percent) |
|---|---|
| New Pacing Indication | 1 (0.1%) |
| ATP | 1 (0.1%) |
| CRT Indication | 1 (0.1%) |
| Ventricular Overdrive Pacing | 1 (0.1%) |
Mortality
The Kaplan-Meier all-cause mortality was 3.2% after 2 years of follow-up, and 4.7% at 3 years.
Results by Patient Enrollment Date
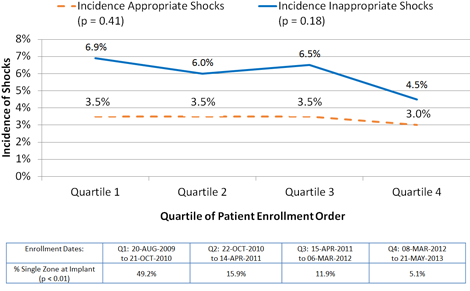
Enrollment date quartiles were used to assess event rates over time. The rate of complications and infections requiring system explantation declined over time.
Six Month Incidence of Complications
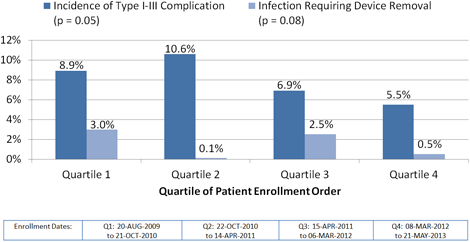
The rate of therapy for VT/VF was similar across quartiles of enrollment in the studies. The most recently implanted quartile of patients had a lower rate of inappropriate shocks at 6 months though the trend did not reach statistical significance (Q1:6.9%, Q2: 6.0%, Q3:6.5%, Q4: 4.5%, p=0.18), while there was a major increase in use of dual zone programming from 51% to 95% (p<0.01)
Six Month Incidence of Appropriate and Inappropriate Shocks