MADIT-CRT Long Term Follow-up
Multicenter Automatic Defibrillator Implantation Trial Cardiac Resynchronization Therapy (MADIT-CRT)
Status: Ongoing
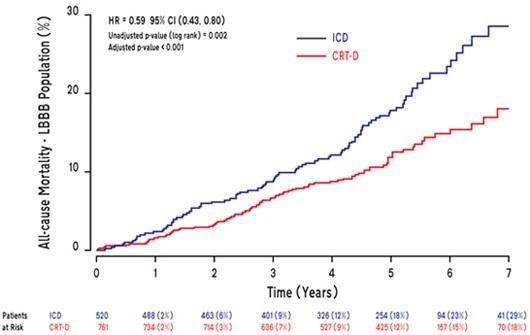
In the MADIT CRT Long-Term Follow-Up study, patients with left bundle branch block (LBBB) who received a CRT-D demonstrated a significant and sustained survival benefit and had a reduction in first heart failure events compared to patients who received an ICD.
- The relative risk of mortality was reduced by 41% in LBBB patients who received CRT-D compared to ICD
- This mortality reduction corresponds to a number needed to treat (NNT) of 9; or 1 life saved over 7 years for every 9 patients who receive a CRT-D
- Over 80% of patients who received CRT therapy were still alive at 7 years
- CRT-D patients had a 62% reduction in the relative risk of a first heart failure event compared to patients who did not receive CRT
- Early intervention in indicated patients can reduce death and increase the life span of patients
- Boston Scientific has responded to longer patient survival by developing CRT defibrillators with the longest projected battery longevity in the industry (LongLasting)
- The longer lasting CRT-D devices from Boston Scientific can reduce the need for procedures in patients with heart failure, and ultimately reduce health care costs and device infections