Promus PREMIER™
Everolimus-Eluting Platinum Chromium Coronary Stent System
Features the first customized Platinum Chromium Stent architecture, the market-leading Everolimus drug and PVDF-HFP polymer combination and an enhanced catheter to provide PREMIER outcomes.
Key Resources
Explore
Product Details
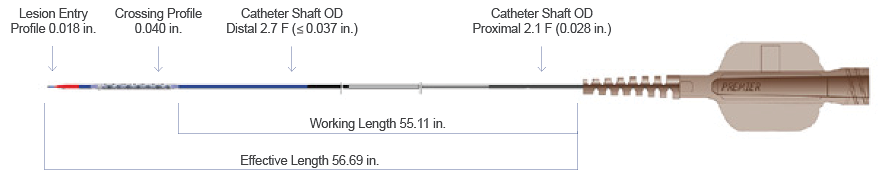
Ordering Information
| MONORAIL | ||||
|---|---|---|---|---|
| (mm) | STENT LENGTH (mm) | |||
| 8 | 12 | 16 | 20 | |
| 2.25 | H7493952808220 | H7493952812220 | H7493952816220 | H7493952820220 |
| 2.5 | H7493952808250 | H7493952812250 | H7493952816250 | H7493952820250 |
| 2.75 | H7493952808270 | H7493952812270 | H7493952816270 | H7493952820270 |
| 3 | H7493952808300 | H7493952812300 | H7493952816300 | H7493952820300 |
| 3.5 | H7493952808350 | H7493952812350 | H7493952816350 | H7493952820350 |
| 4 | H7493952808400 | H7493952812400 | H7493952816400 | H7493952820400 |
| (mm) | STENT LENGTH (mm) | |||
|---|---|---|---|---|
| 24 | 28 | 32 | 38 | |
| 2.25 | H7493952824220 | H7493952828220 | H7493952832220 | N/A |
| 2.5 | H7493952824250 | H7493952828250 | H7493952832250 | H7493952838250 |
| 2.75 | H7493952824270 | H7493952828270 | H7493952832270 | H7493952838270 |
| 3 | H7493952824300 | H7493952828300 | H7493952832300 | H7493952838300 |
| 3.5 | H7493952824350 | H7493952828350 | H7493952832350 | H7493952838350 |
| 4 | H7493952824400 | H7493952828400 | H7493952832400 | H7493952838400 |
Clinical Information
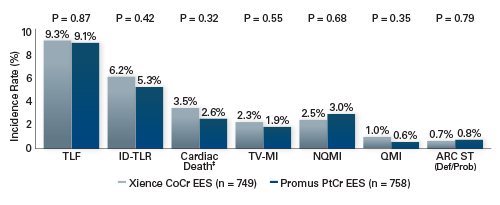
PLATINUM Workhorse Trial
Study Objective
Evaluate the safety and effectiveness of the Promus PtCr EES Coronary Stent System* for the treatment of patients with up to 2 de novo lesions ≤ 24 mm in length; ≥ 2.50 mm to ≤ 4.25 mm in diameter compared to the Xience CoCr EES
Study Design
Prospective, Randomized, Controlled, Non-inferiority, Multicenter
Economic Value
Helping You Save More
Our unique Platinum Chromium (PtCr) Stent alloy can help reduce device usage, procedure times and radiation exposure.
Learn HowReimbursement
The C-Code used for the Promus PREMIER Everolimus-Eluting Platinum Chromium Coronary Stent System is C1874 Stent, coated/covered with delivery system. C-Codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific Corporation is not responsible for correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.
Tools and Resources
-
 Apr 02, 2014
Apr 02, 2014Latex Information: Promus PREMIER Everolimus-Eluting Platinum Chromium Coronary Stent System
PDF, 192.0 KB
-
 Apr 02, 2014
Apr 02, 2014Metal Composition: Promus PREMIER Everolimus-Eluting Platinum Chromium Coronary Stent System
PDF, 268.0 KB
-
 Apr 02, 2014
Apr 02, 2014MRI Safety: Promus PREMIER Everolimus-Eluting Platinum Chromium Coronary Stent System
PDF, 208.0 KB