Lynx™
Suprapubic Mid-Urethral Sling System
Intended to facilitate suprapubic device passage with an association mechanism that is designed for smooth transition from loop to mesh.
Key Resources
Explore
Product Details
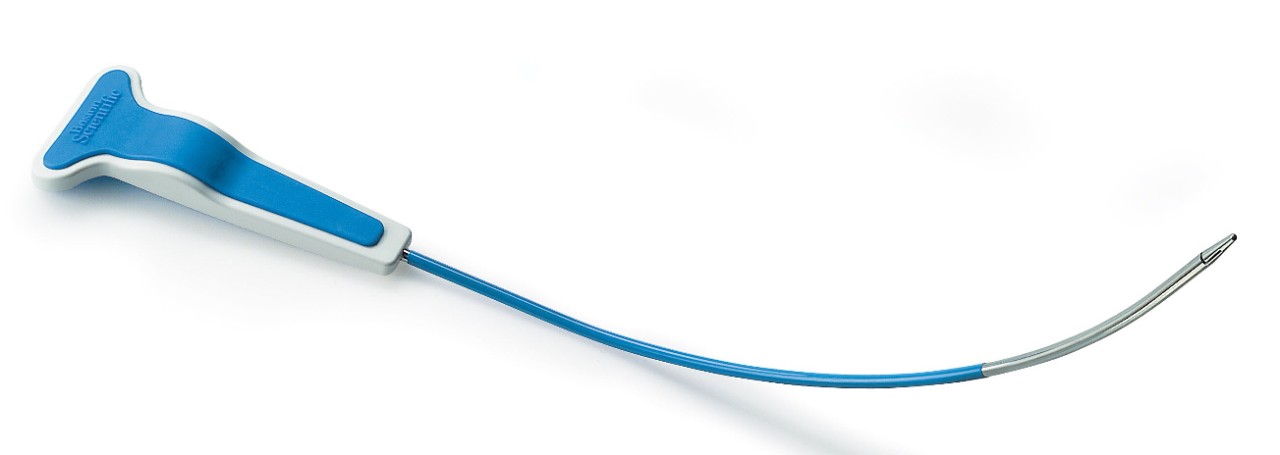
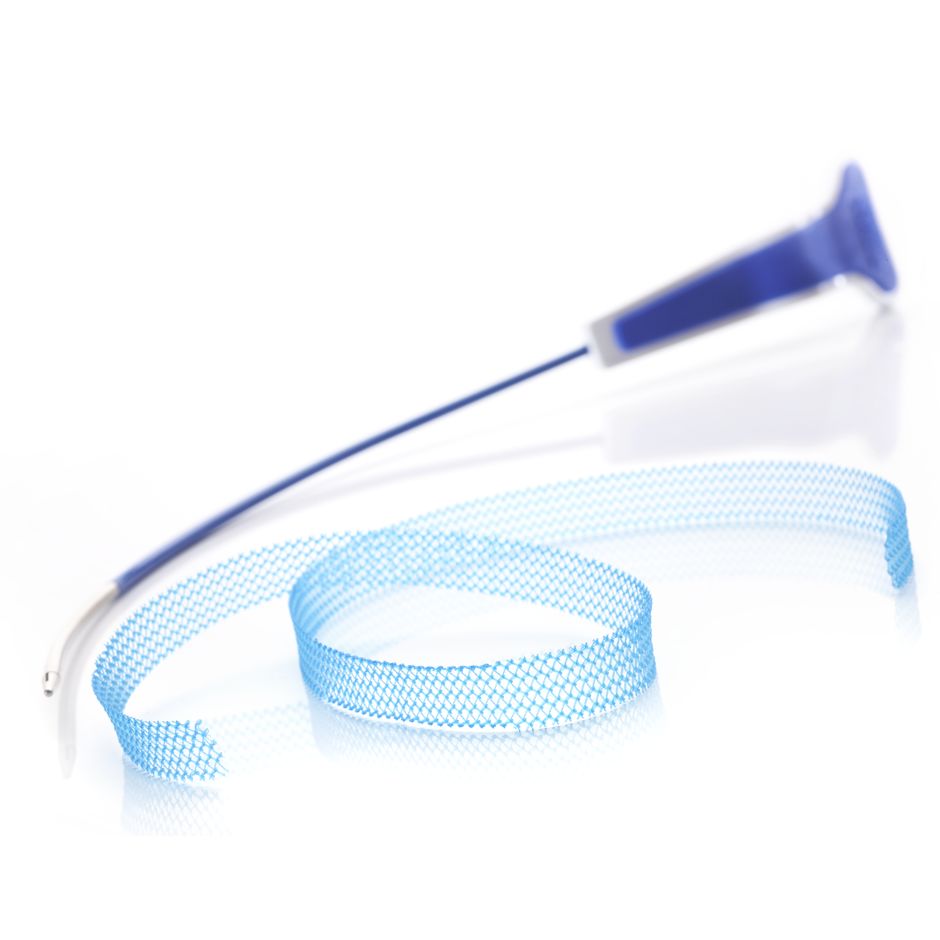
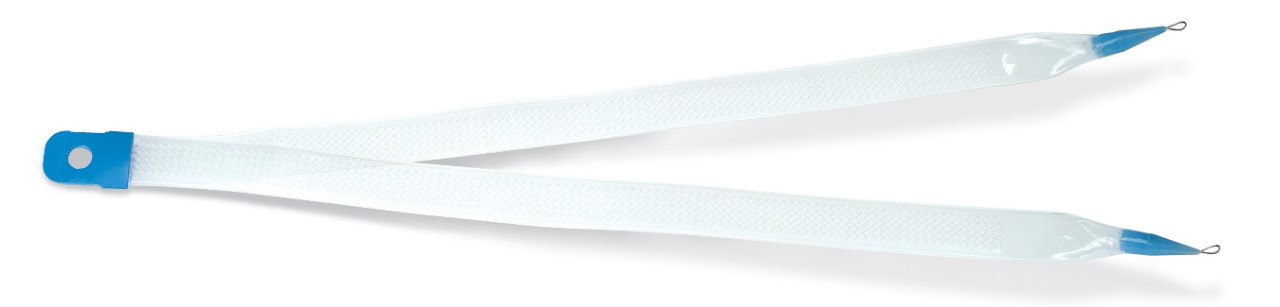
Handle
- Non-skid grip is designed to prevent hand from slipping during intra-operative manipulations
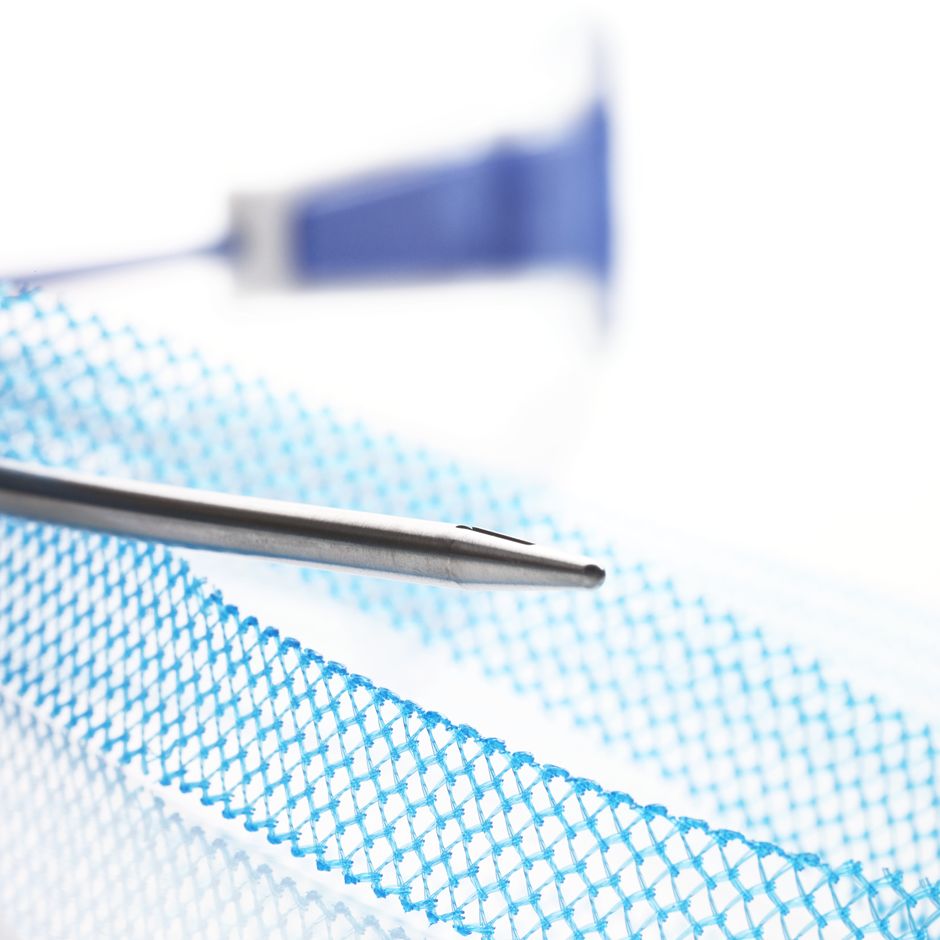
Needle
- Designed to facilitate suprapubic device passage
- Needle is colored to enhance visibility during cystoscopy
Advantage™ Mesh
Centering Tab
- Designed for proper alignment of the center of the mesh under the urethra
- Allows the physician to apply counter tension to the sling while preserving the mesh integrity
Association Loop
- Facilitates needle engagement and removal
- Designed for smooth transition from loop to mesh allowing for minimal disruption to tissue
Removable Engagement Mechanism
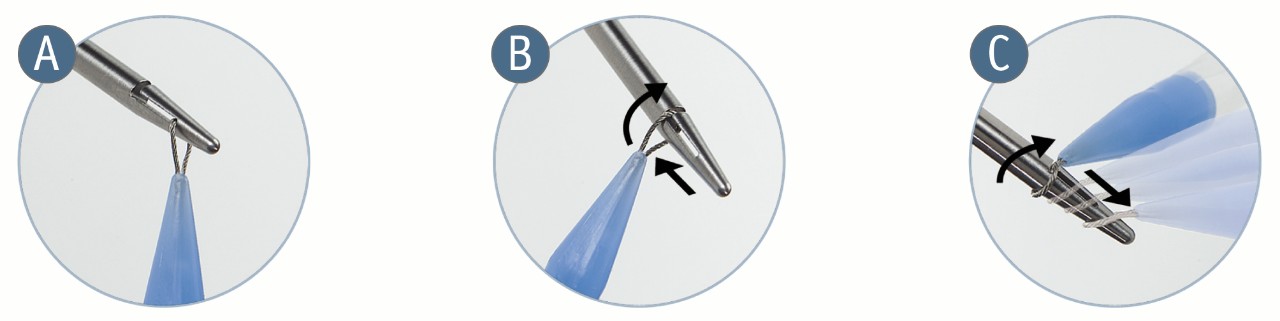
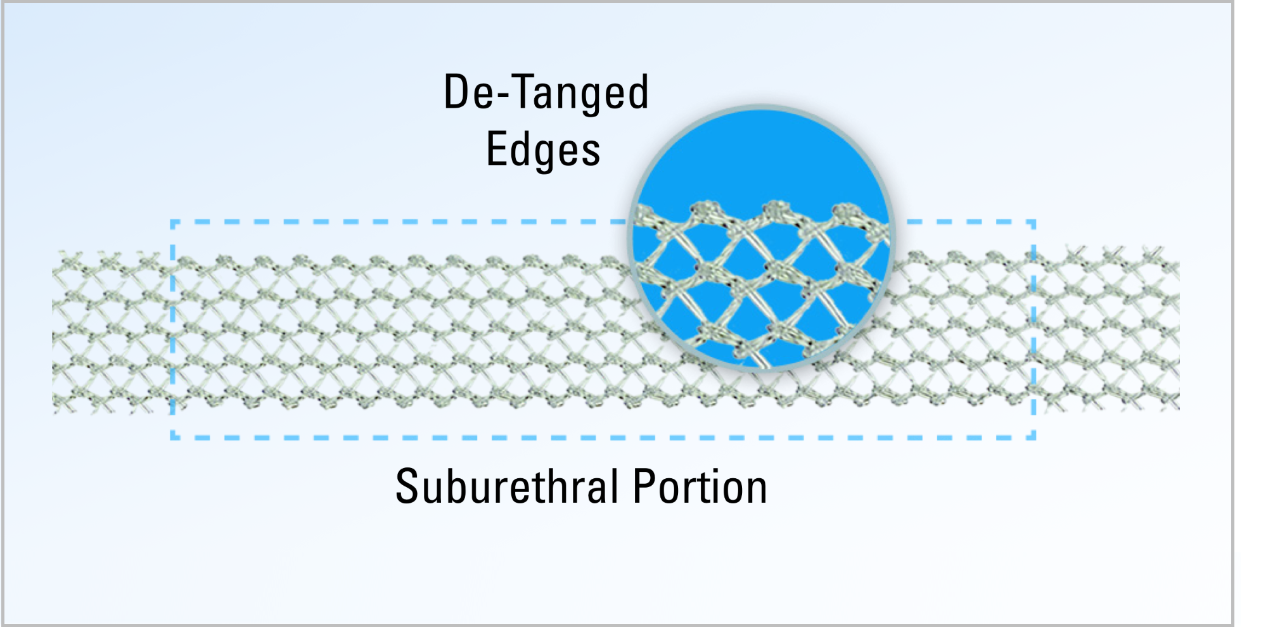
De-Tanged Polypropylene Material
Ordering Information
| Order Number | UPN | Description | Quantity |
|---|---|---|---|
| 850301 | M0068503010 | Lynx Blue Sling System | 2 Delivery Devices and 1 Mesh Assembly |
| 850300 | M0068503000 | Lynx Sling System | 2 Delivery Devices and 1 Mesh Assembly |