Leads
All Specialties (-)
-
ACUITY™ X4 Quadripolar LV Leads
New Solutions.
Meaningful Outcomes.
ACUITY™ X4 Quadripolar LV leads are the first and only LV leads uniquely designed to promote non-apical pacing options, helping physicians to pace from an optimal site for improved CRT response.- Electrophysiology
-
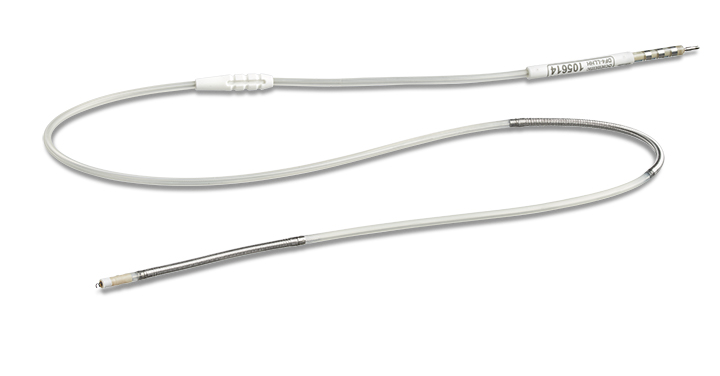
INGEVITYTM+ Pacing Lead
Proven Longevity.
Predictable Performance.
INGEVITY+ is built on the proven INGEVITY™ platform, with nearly 700,000 INGEVITY leads sold worldwide with a 99.2% survival at year 7.1
This active fixation pacing lead is designed for predictable performance.- Electrophysiology
-
INGEVITY™ MRI Pacing Lead
INGEVITY MRI Pacing Leads are passive models that can be used as part of the IMAGEREADY™ MR-Conditional Pacing System for safe and effective scanning in 1.5T and 3T MRI environments when MRI Conditions of Use are met.1
- Electrophysiology
-
RELIANCE 4-FRONT™ Pace/Sense and Defibrillation Lead
RELIANCE 4-FRONT is built on the RELIANCE™ platform, leveraging our 25-year legacy of the industry's most reliable ICD leads. Experience the difference in lead handling and performance with enhancements inspired by physicians.
- Electrophysiology
-
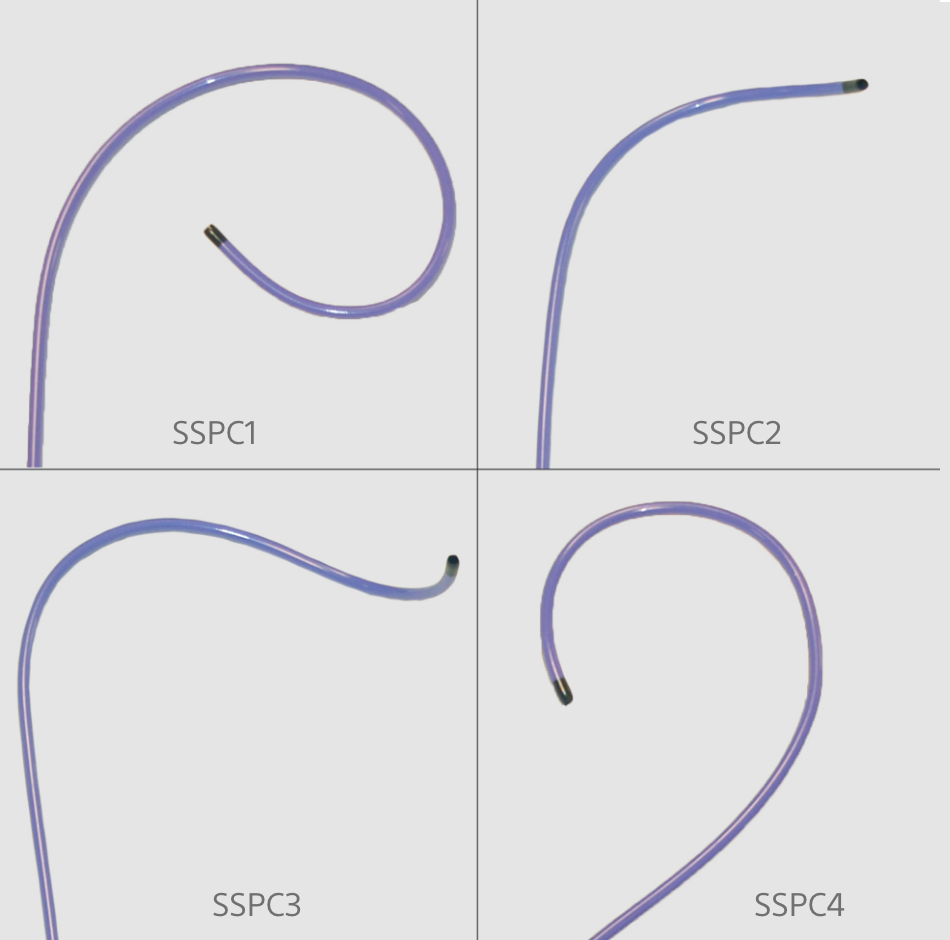
SSPC SITE SELECTIVE PACING CATHETERS
The SSPC or Site Selective Pacing Catheter family are single-use percutaneous catheters intended to introduce various types of catheters and pacing or defibrillator leads.
The SSPC Catheters have been used in human clinical cases since 2019, increasing substantially in the last few years. Over 20,000 SSPC Catheters have been shipped for use in the USA since 2019.- Electrophysiology