EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
The Only Device of its Kind
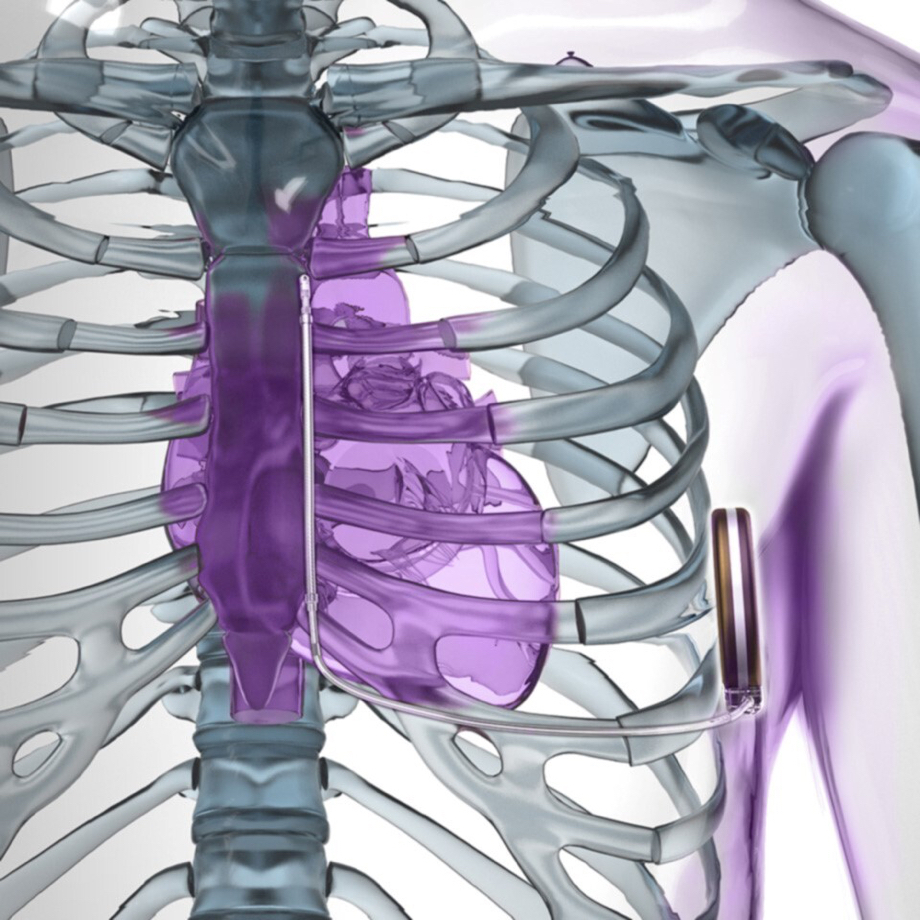
The EMBLEM MRI S-ICD is the only subcutaneous implantable defibrillator system that provides protection from both sudden cardiac death and the risks and complications associated with transvenous leads.
Key Resources
Explore
Product Details
The EMBLEM MRI S-ICD system provides effective defibrillation without transvenous leads, offering a less invasive solution for patients at risk of sudden cardiac arrest.
- Preserves the vasculature
- Eliminates potential for vascular injury, transvenous lead insertion complications, lead-associated tricuspid regurgitation, mechanically induced pro-arrhythmia, and transvenous lead failure and associated extraction risk
- Remains outside the sternum, never touching the heart
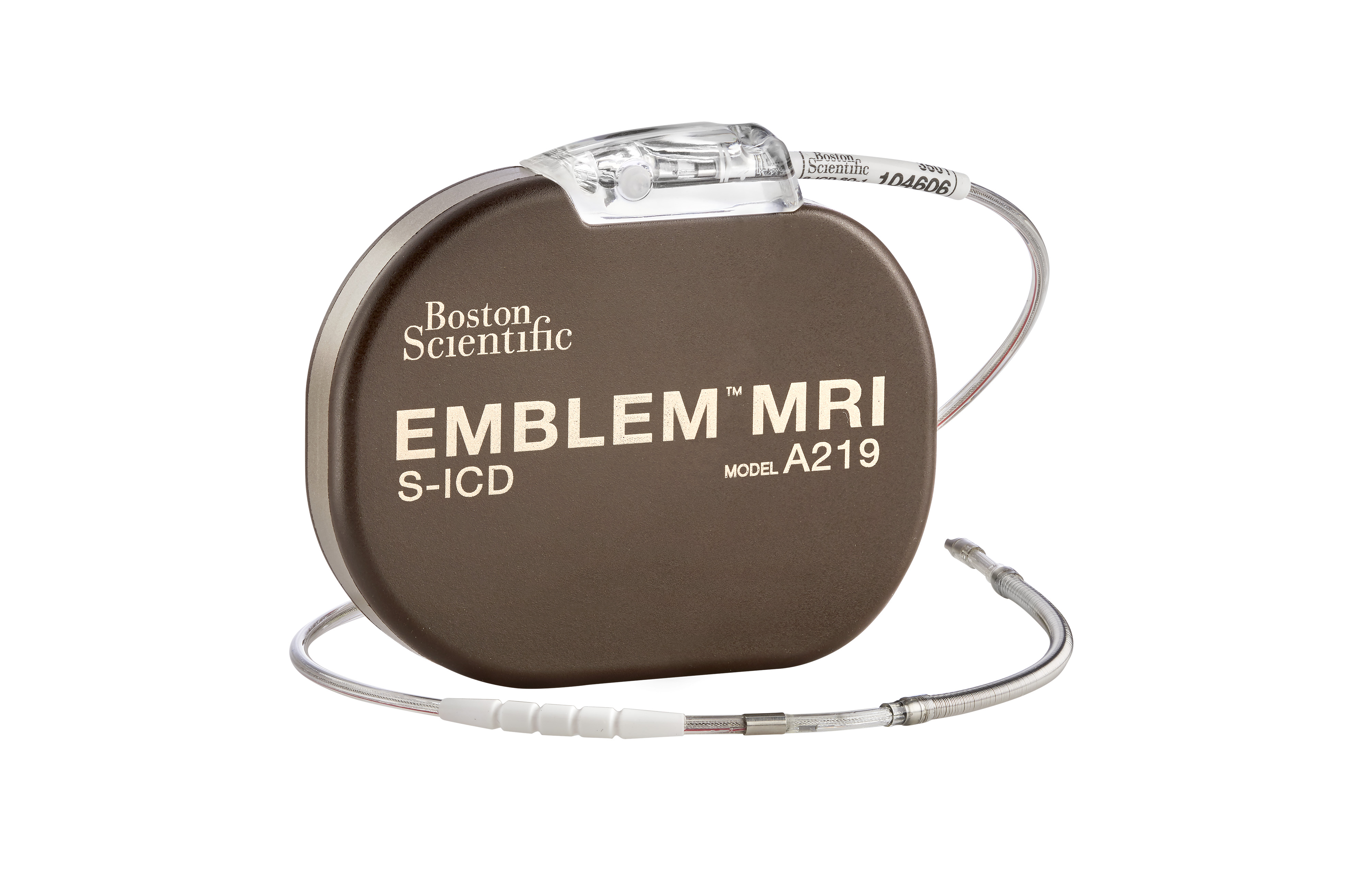
| EMBLEM MRI S-ICD Pulse Generator Mechanical Specifications | |
|---|---|
| Model Number | A219 |
| Size (W x H x D) | 83.1 x 69.1 x 12.7 mm |
| Mass | 130 g |
| Volume | 59.5 cc (cm³) |
| Projected Longevity | 8.7 years¹ |
| Battery Chemistry | Boston Scientific Li/MnO2 |
| Warranty | 6 years* |
| Remote Patient Monitoring Capability | Enabled for use with LATITUDE™ NXT remote patient management |
*For full warranty terms and conditions go to www.bostonscientific.com/en-US/pprc/warranty-info-forms.html
Real World Evidence - Battery Longevity¹
Emblem S-ICD longevity has been clinically shown, based on real world evidence and data collected from 32,678 implanted S-ICD devices, to last 8.7 years...with all features turned on.
Click here to access the ESC Abstract on real world battery longevity.

Low Inappropriate Shocks Using Recent Technologies and Implant Technique
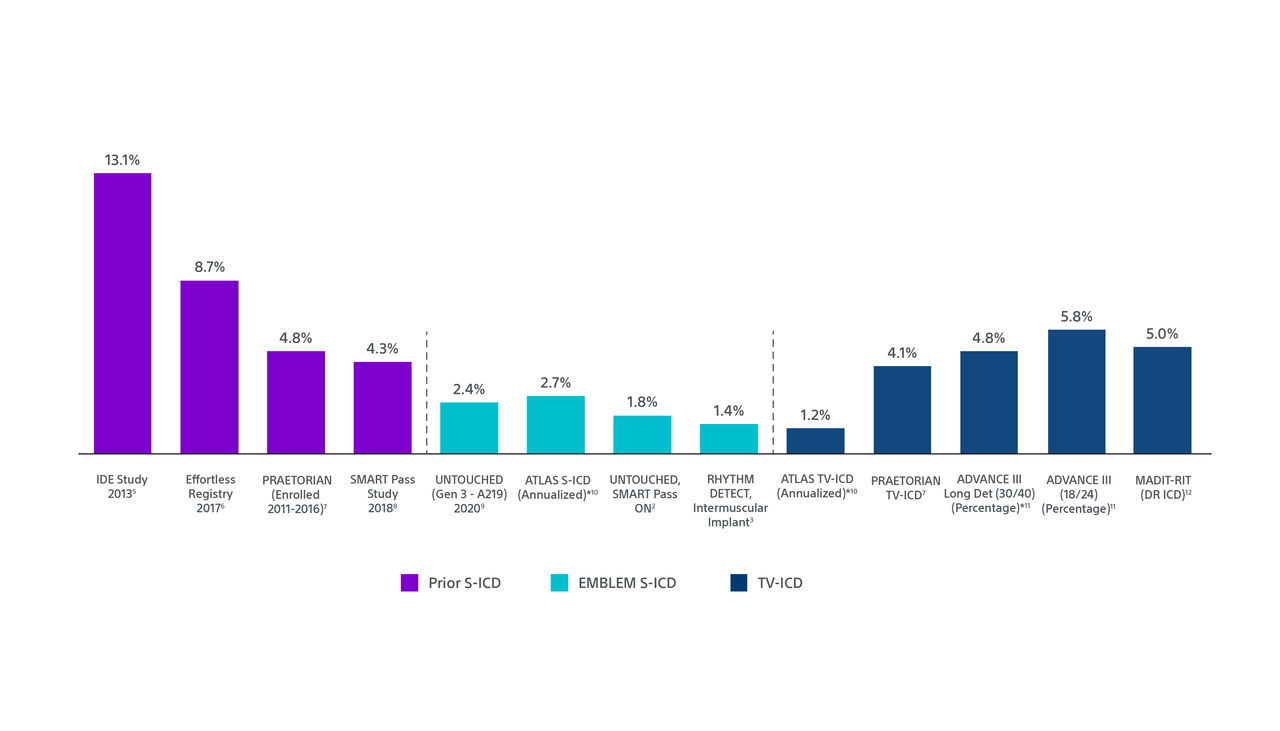
*Rates are calculated using Kaplan-Meier analyses except where indicated.
Results from different clinical studies are not directly comparable and information is provided for educational purposes only.
Ordering Information
| Model Number | Description |
|---|---|
| A219 | EMBLEM MRI S-ICD Pulse Generator |
| 3501 | EMBLEM S-ICD Electrode |
| 4712 | EMBLEM S-ICD Electrode Insertion Tool |
| 3300 | LATITUDE™ Programming System |
Reimbursement
Training & Education

Explore continuing education courses, best practices modules and other training and resources for S-ICD.
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.