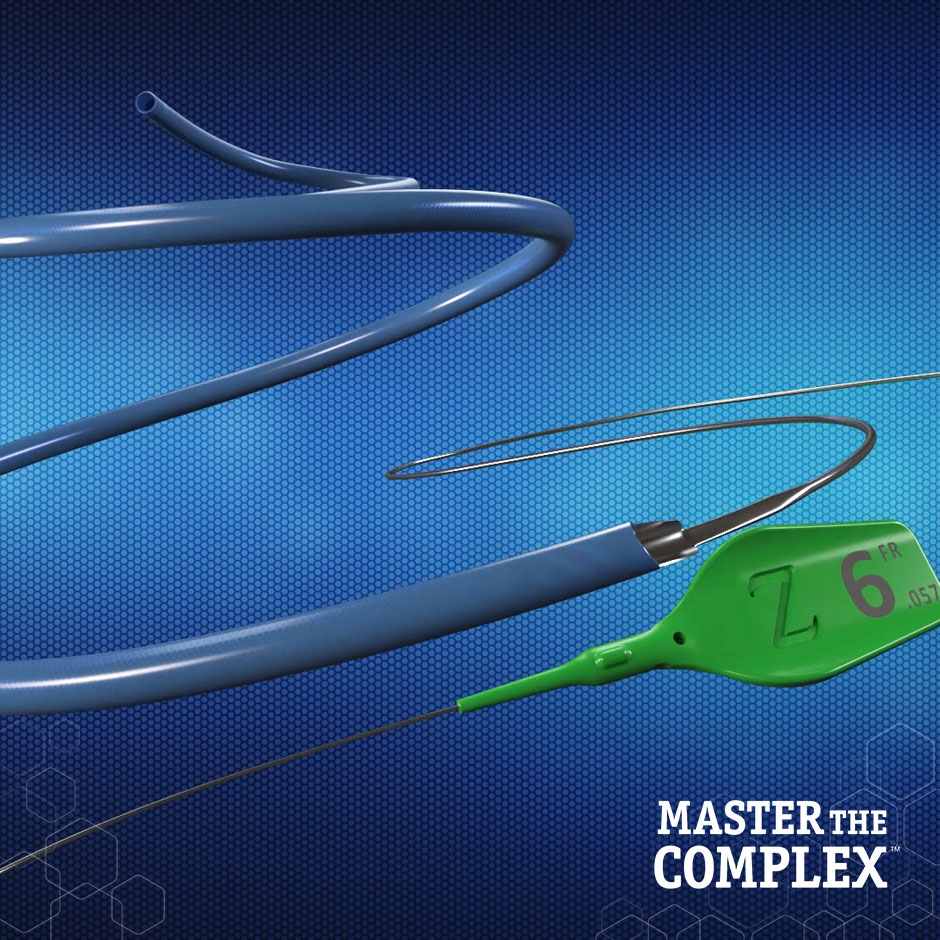
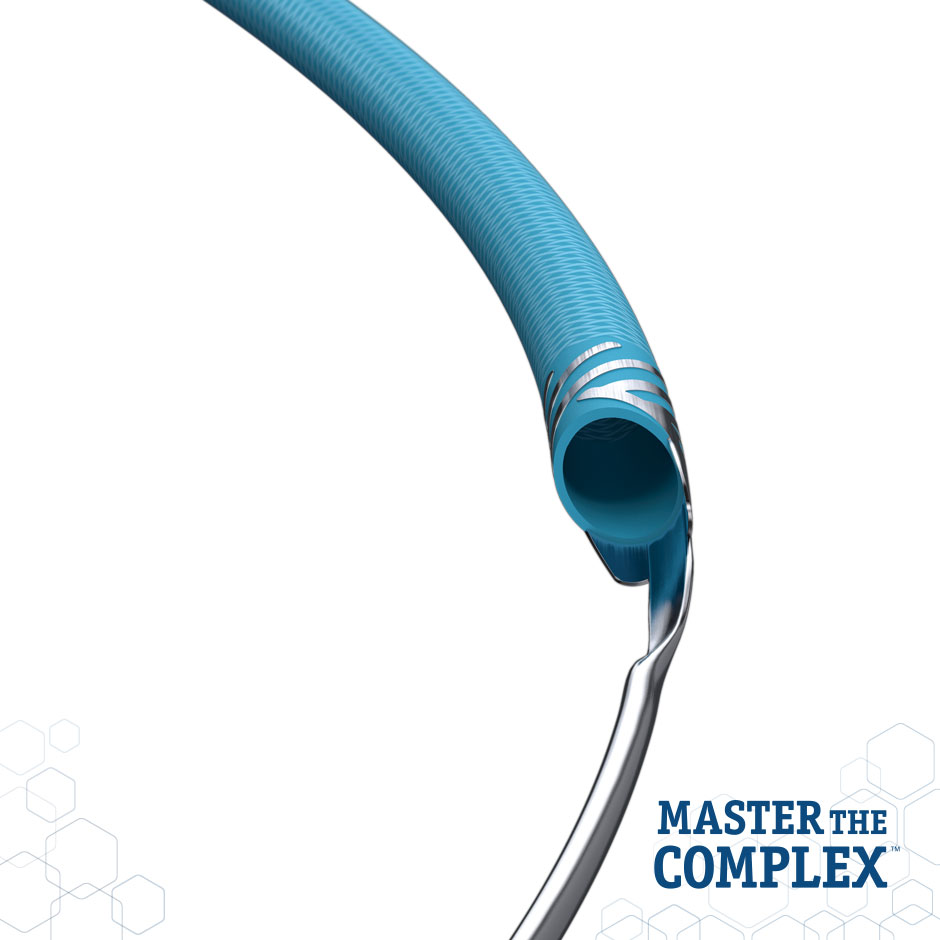
GUIDEZILLA™ II
Guide Extension Catheter
Powerful Reach. Predictable Performance. GUIDEZILLA™ II Guide Extension Catheter provides additional back-up support and facilitates easy delivery of ancillary devices.
Explore
Product Details
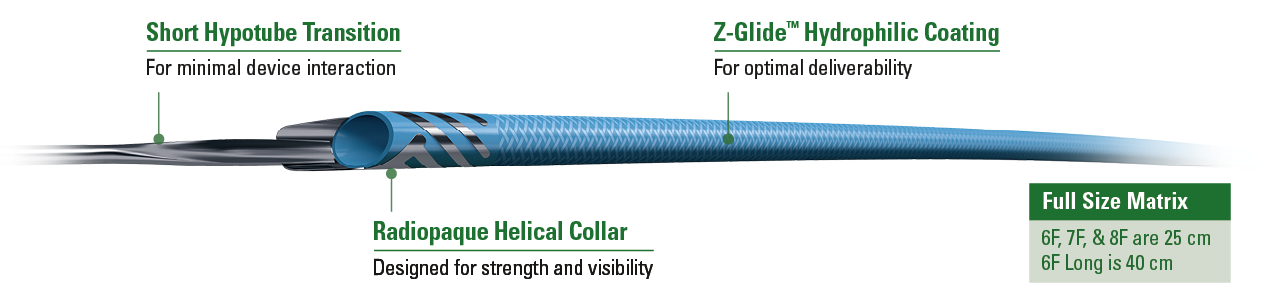
Design Features
| Feature | Metric | Benefit |
|---|---|---|
| Sizes | 6F, 7F, 8F, AND 6F Long | Broad Size Matrix |
| Guide Segment | 25 cm on 6F, 7F, 8F (40 cm on 6F Long) | 40 cm 6F Long Designed for TRI |
| Working Length | 150 CM | Elongated Proximal Hypotube Shaft |
| Collar | Platinum Iridium Helical Collar | Optimal Visibility and Smooth Device Interaction |
| Coating | Z-Glide™ | Hydrophilic Coating Aids Deliverability |
| Radiopaque | Distal Marker Band Radiopaque Collar | Highly visible |
| Hypotube Transition | 6 mm | Optimized to Reduce |
| Hub Design |  | Ergonomic and Easily Identifiable |
Ordering Information
| Size | GTIN | Ref/Catalog Number | Compatible Guide Catheter | Inner Diameter | Outer Diameter |
|---|---|---|---|---|---|
| 6F | 08714729939450 | H7493933515060 | 6F I.D. ≥0.070" (1.78mm) | 0.057" (1.45 mm) | 0.067" (1.71 mm) |
| 6F LONG (40 cm) | 08714729939467 | H74939335150610 | 6F I.D. ≥0.070" (1.78 mm) | 0.057" (1.45 mm) | 0.067" (1.71 mm) |
| 7F | 08714729939474 | H7493933515070 | 6F I.D. ≥0.078" (1.98 mm) | 0.063" (1.60 mm) | 0.073" (1.86 mm) |
| 8F | 08714729939481 | H7493933515080 | 6F I.D. ≥0.088" (2.24 mm) | 0.072" (1.83 mm) | 0.083" (2.11 mm) |