A bold step forward.
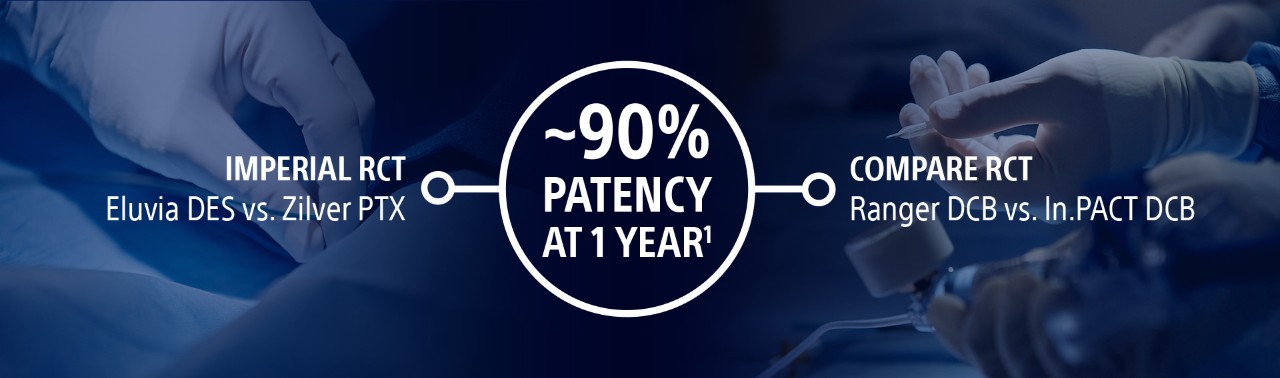
Boston Scientific is committed to advancing science in the fight against PAD by boldly innovating with next-generation, drug-eluting technology. Backed by Level-1 Randomized Controlled Trials, our proven exceptional results put the power of choice in the hands of those who make it happen. Together, we can save more limbs and help more people walk without pain.
Investment in clinical data
The best outcomes begin with our investment in the quality and quantity of evidence you demand.